Frequently Asked Questions About Medical Device INVIMA Regulatory Registration, Legal Representation, and Importer of Record (IOR) in Colombia
INVIMA Medical Device Registration and Market Clearance in Colombia
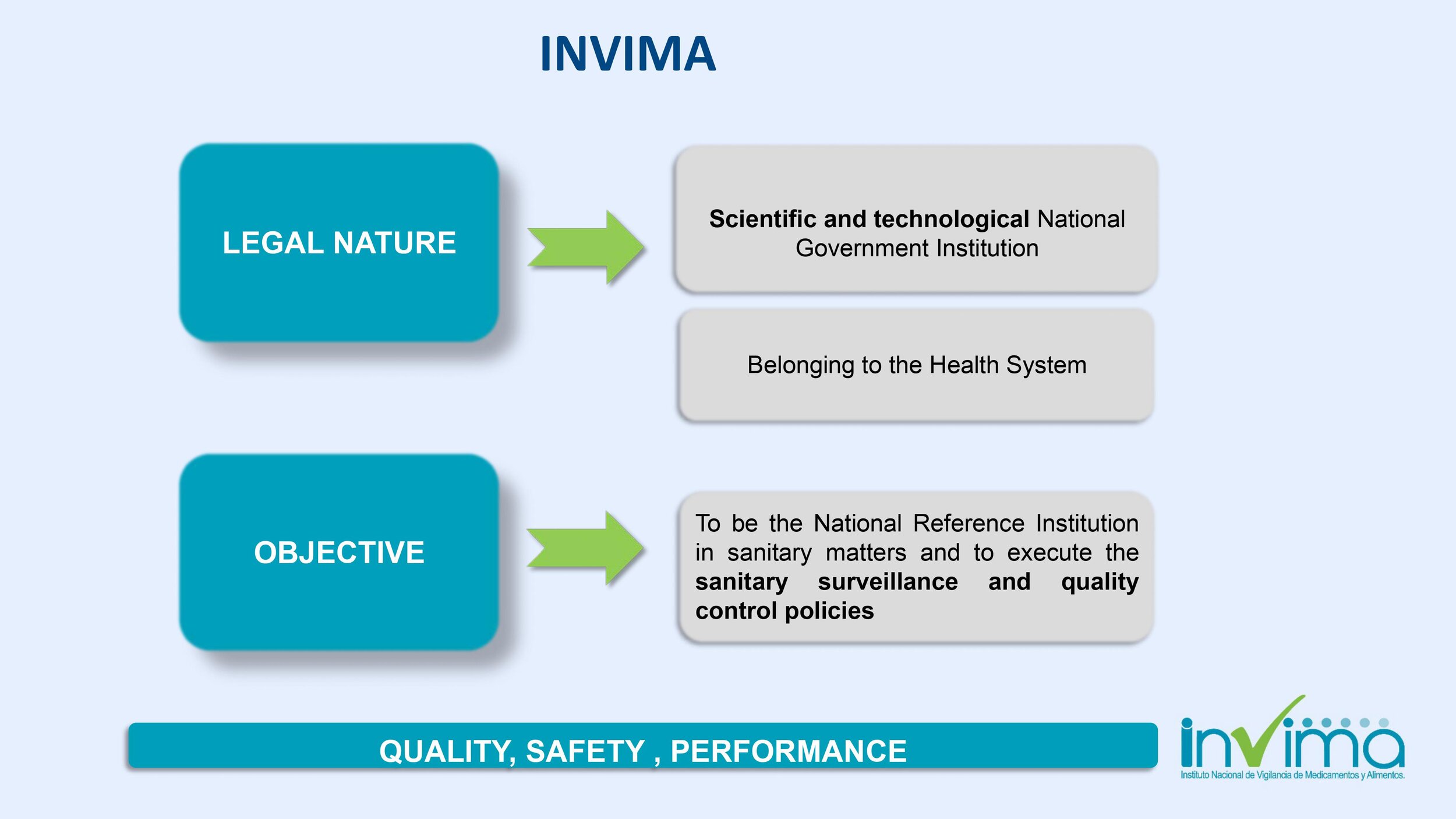
INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) is Colombia's regulatory agency. It was established by the Colombian Ministry of Health to inspect and supervise the production and marketing of health products in the country. The agency is a leader in the region and holds level four status with the World Health Organization (WHO).
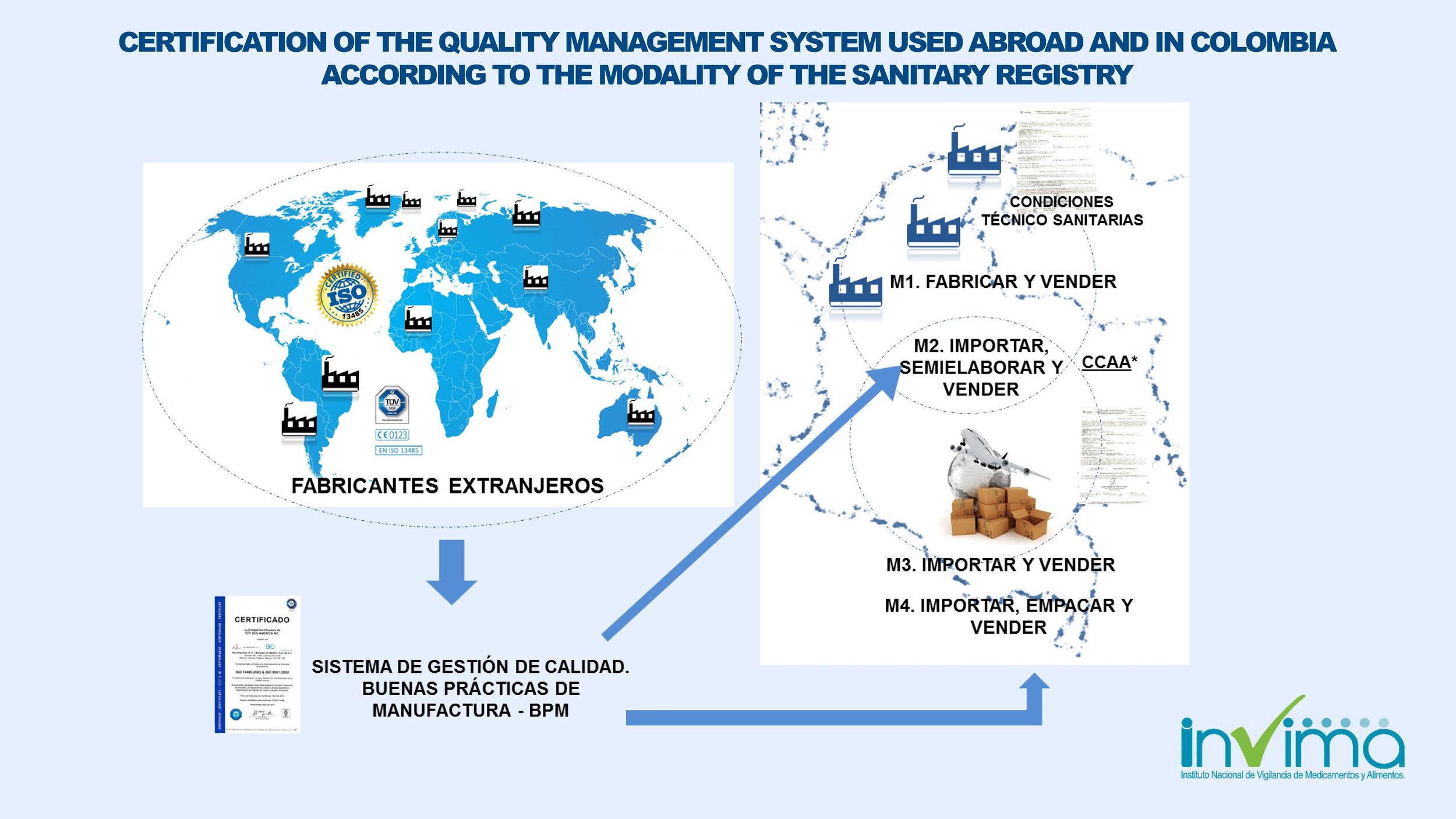
INVIMA regulates Colombia's medical device market. In order to market your device in Colombia, you must obtain sanitary registration (aka marketing authorization or registro sanitario in Spanish) from INVIMA.
INVIMA requires that your medical device is approved in a GHTF-founding member country (i.e., Australia, Canada, European Union, Japan, and the United States of America) or from a country that has an existing regulatory agreement of mutual recognition with Colombia. Foreign manufacturers have the option to have the INVIMA registration certificate issued to their company names and maintain control over their registrations in the Colombian medical device market (they will be able to add or remove at will the name of the importer of record (IOR) listed in the certificate).
bioaccess™ importer of record (IOR) service allows foreign medical device and IVD companies to expand into Colombia with ease. Our IOR service simplifies the market clearance of your medical product at INVIMA and saves you from the costs of establishing a legal entity or from waiting to find a suitable distributor.
Pre-Submission
What's Colombia's regulatory agency?
Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA): www.invima.gov.co
What are the steps to register a medical device in Colombia?
Once you determine the correct classification of your medical device, you must complete the following steps to bring your device to market in Colombia.
Appoint an in-country representative, such as a Legal Representative, if you have no local presence in Colombia.
Obtain a Certificate of Free Sale (CFS) or Certificate to Foreign Government (CFG) from your home country or an INVIMA recognized market/reference country (i.e., USA, Canada, EU, Japan, Australia).
Provide a quality system certificate, such as ISO 13485.
Provide product information and the commercial history of the product. INVIMA will require test reports for Class IIa, IIb and III devices, and clinical data for Class IIb and Class III devices.
Submit these materials to INVIMA in Spanish and pay the required application fee.
Once approved, INVIMA will issue a registration certificate.
Read our blog for a Colombia overview of the regulatory framework for medical devices here.
Is there an expedited INVIMA approval process for lower-risk devices?
INVIMA allows immediate acceptance of Class I and IIa device submissions. The full technical file must still be submitted for review by INVIMA, but certificate issuance is immediate and manufacturers can begin importing right away. Once the formal review begins, the manufacturer must respond to INVIMA’s requests in order to maintain the registration. This allowance was implemented to help address the long review times and large backlog experienced within INVIMA.
Class IIb and III devices are not eligible for this process and must wait until formal review and approval are complete, generally within three to six months, to begin selling.
Can I register my medical device in Colombia under my foreign company's name?
Yes. Your foreign company can be the titleholder of the INVIMA registration certificate. You can have your INVIMA registration certificate issued under your foreign company's name. You don't need to name a local entity as the titleholder of your registration certificate.
Where can I find an overview of Colombia's medical device and in vitro diagnostic (IVD) regulatory environment?







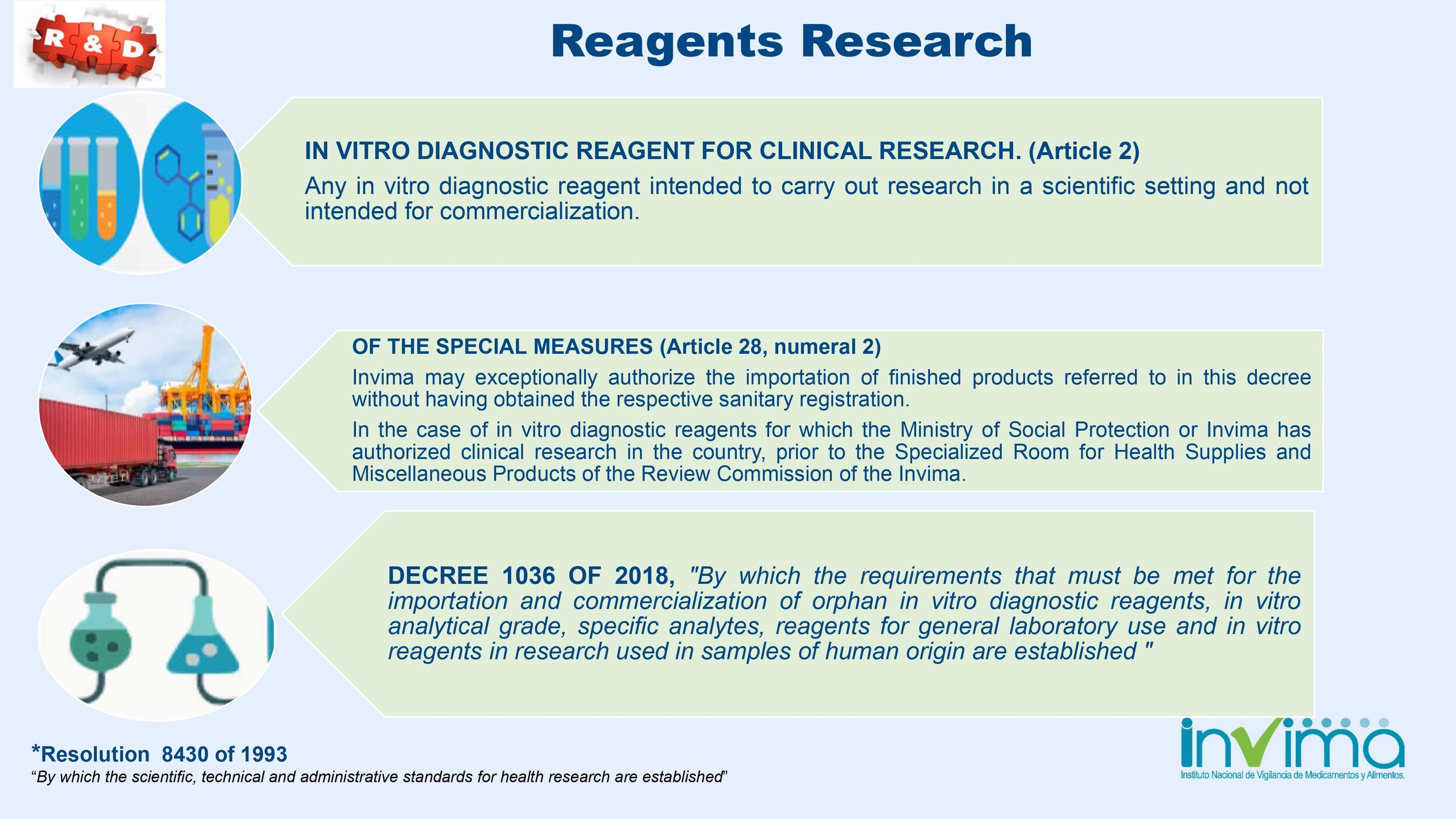
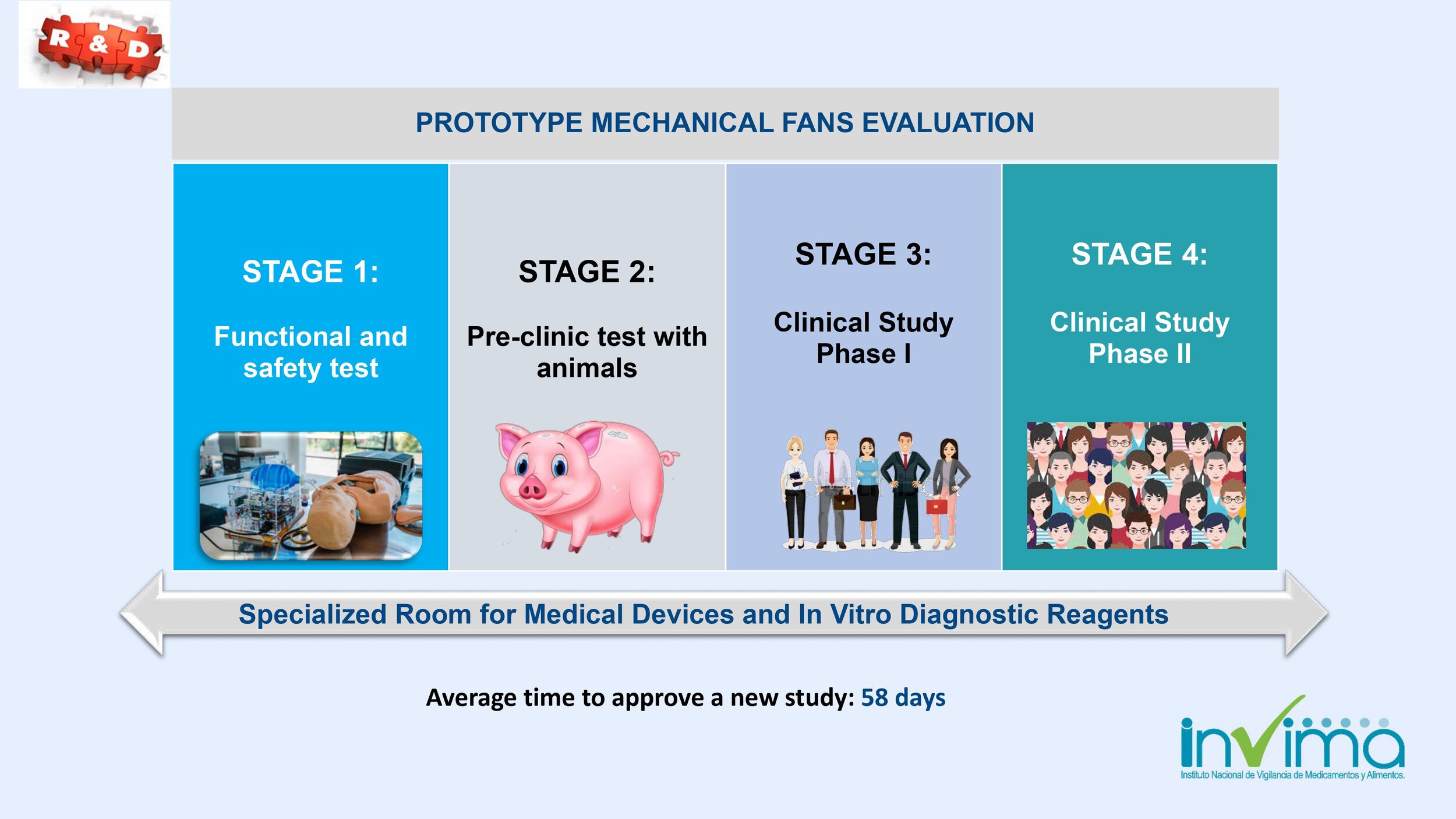
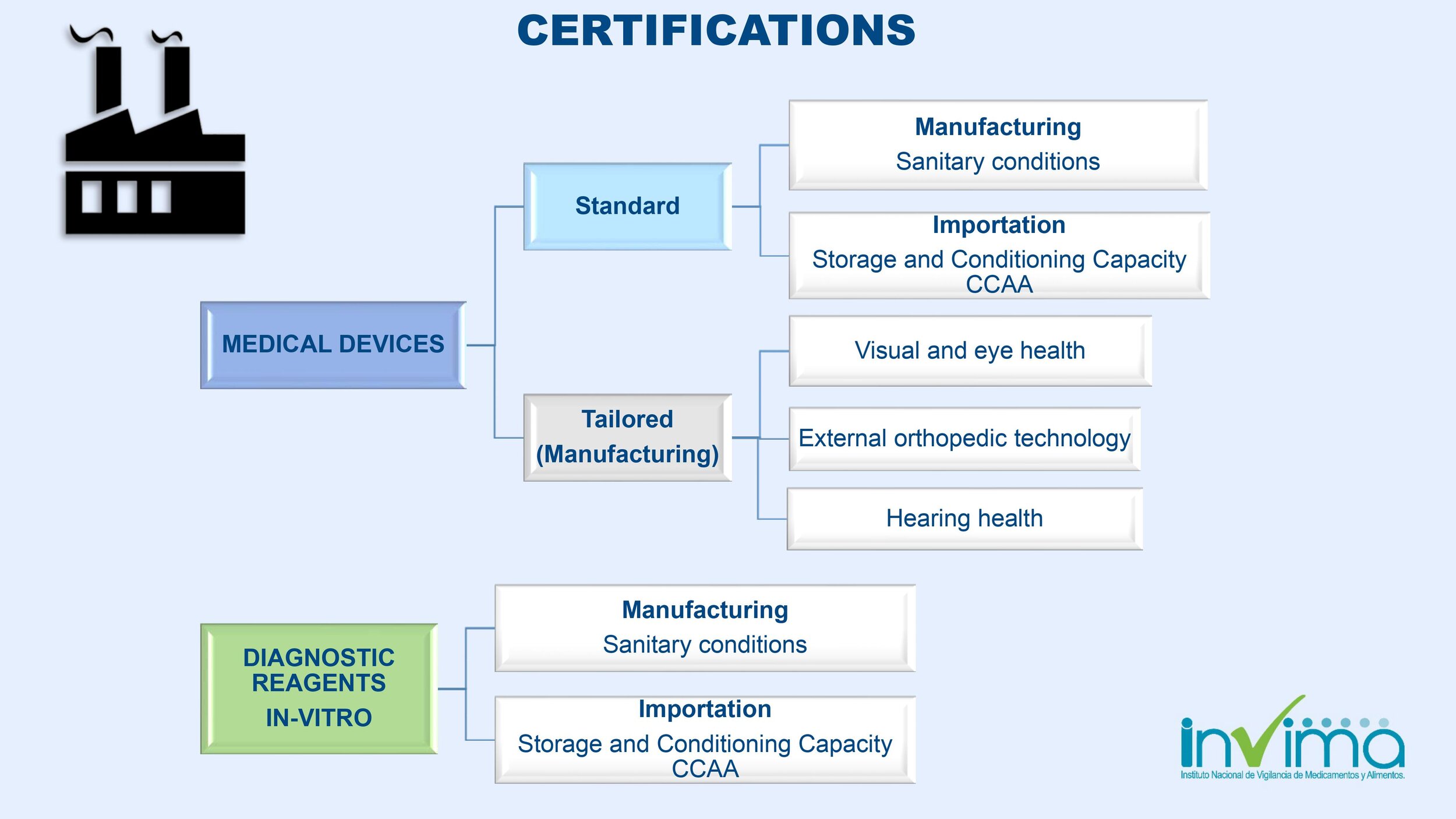
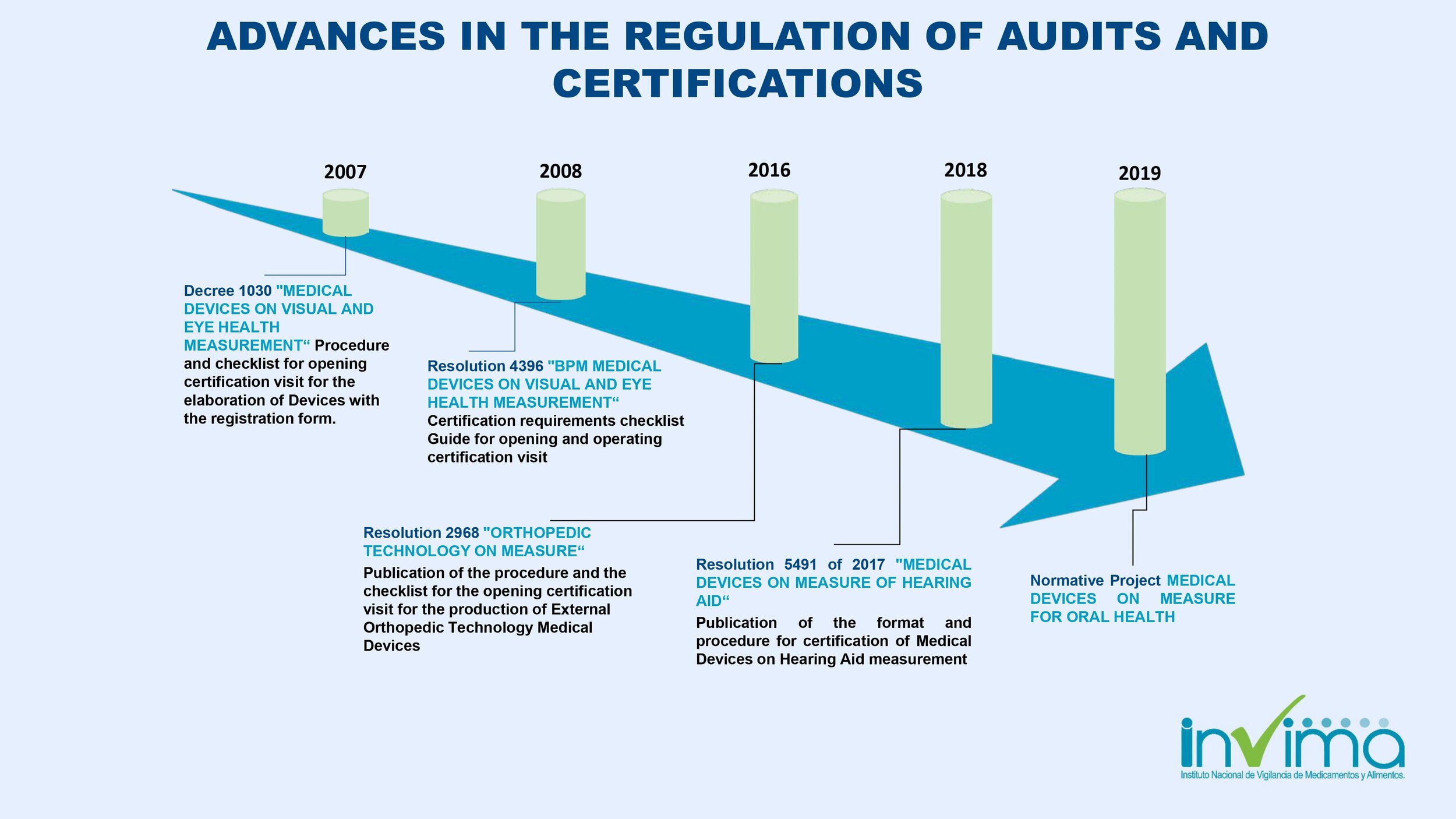
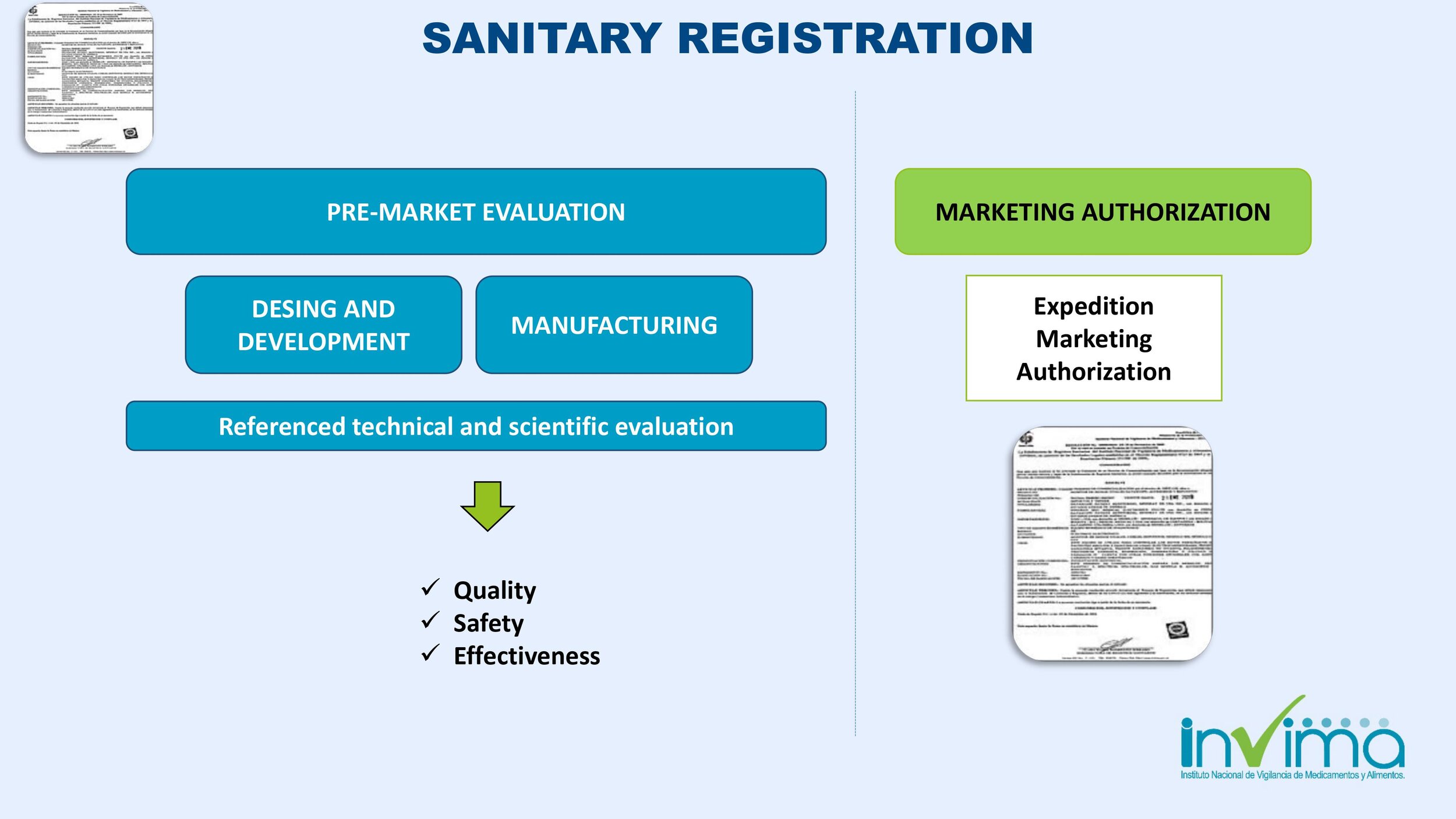

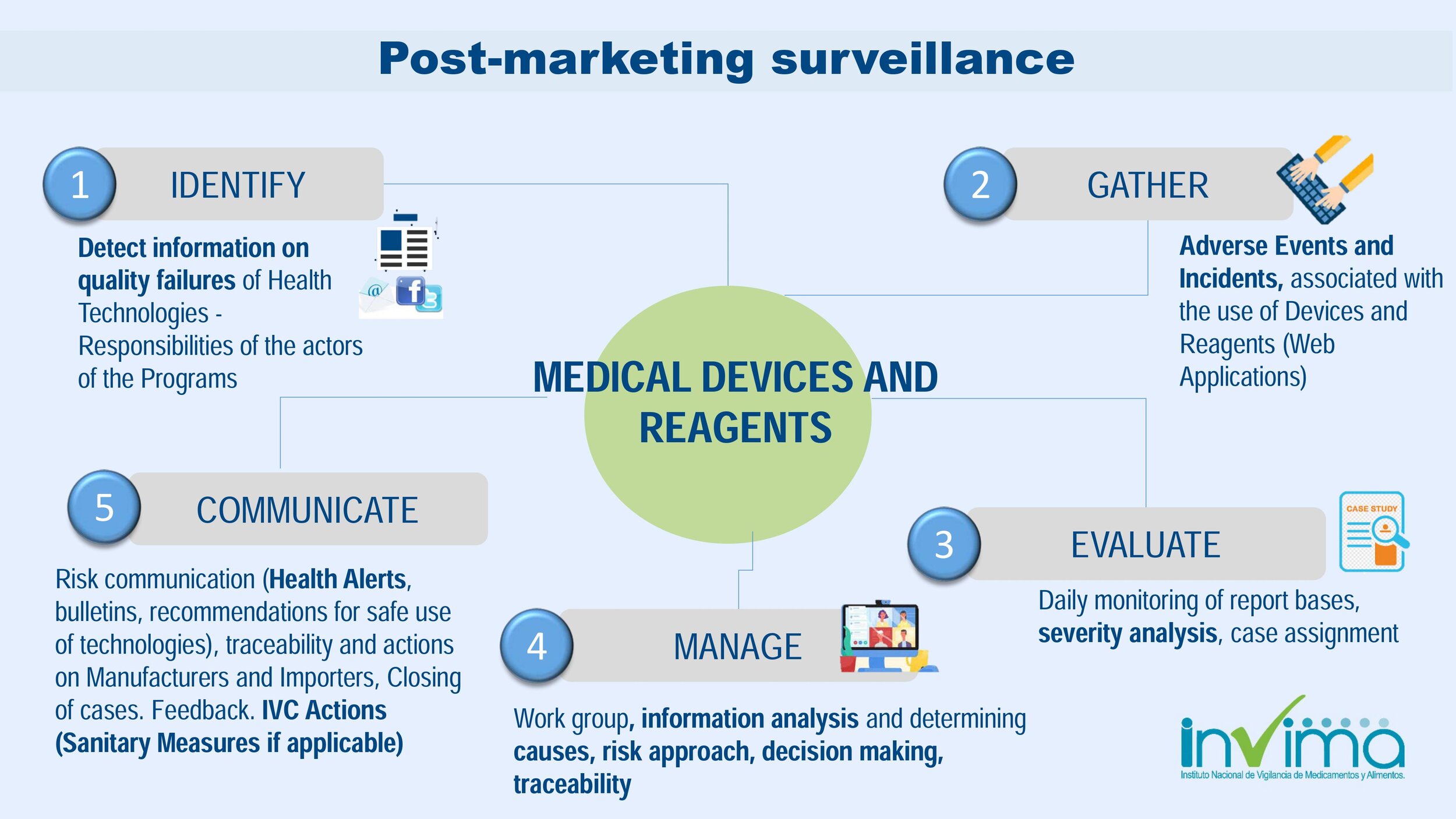
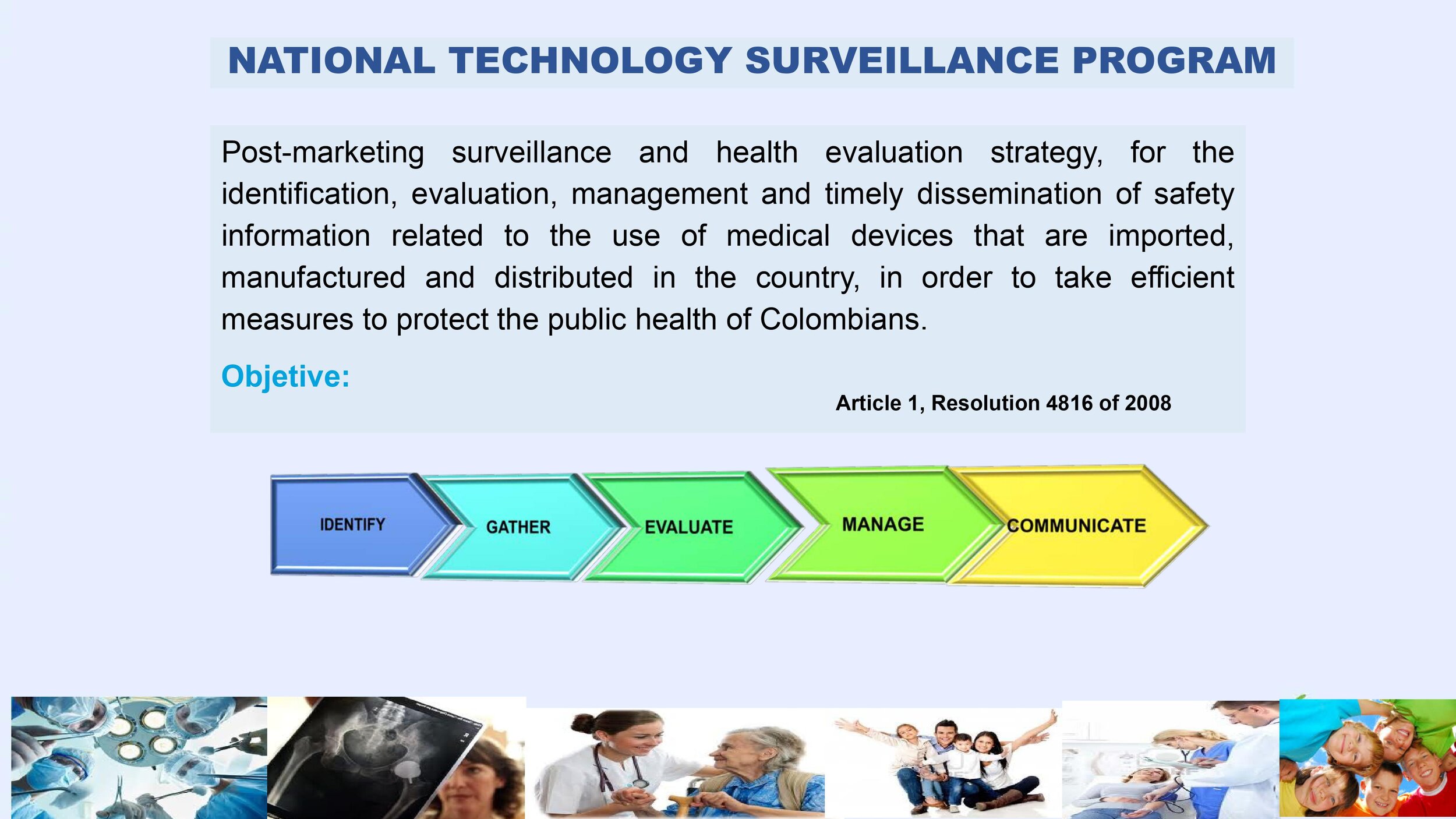

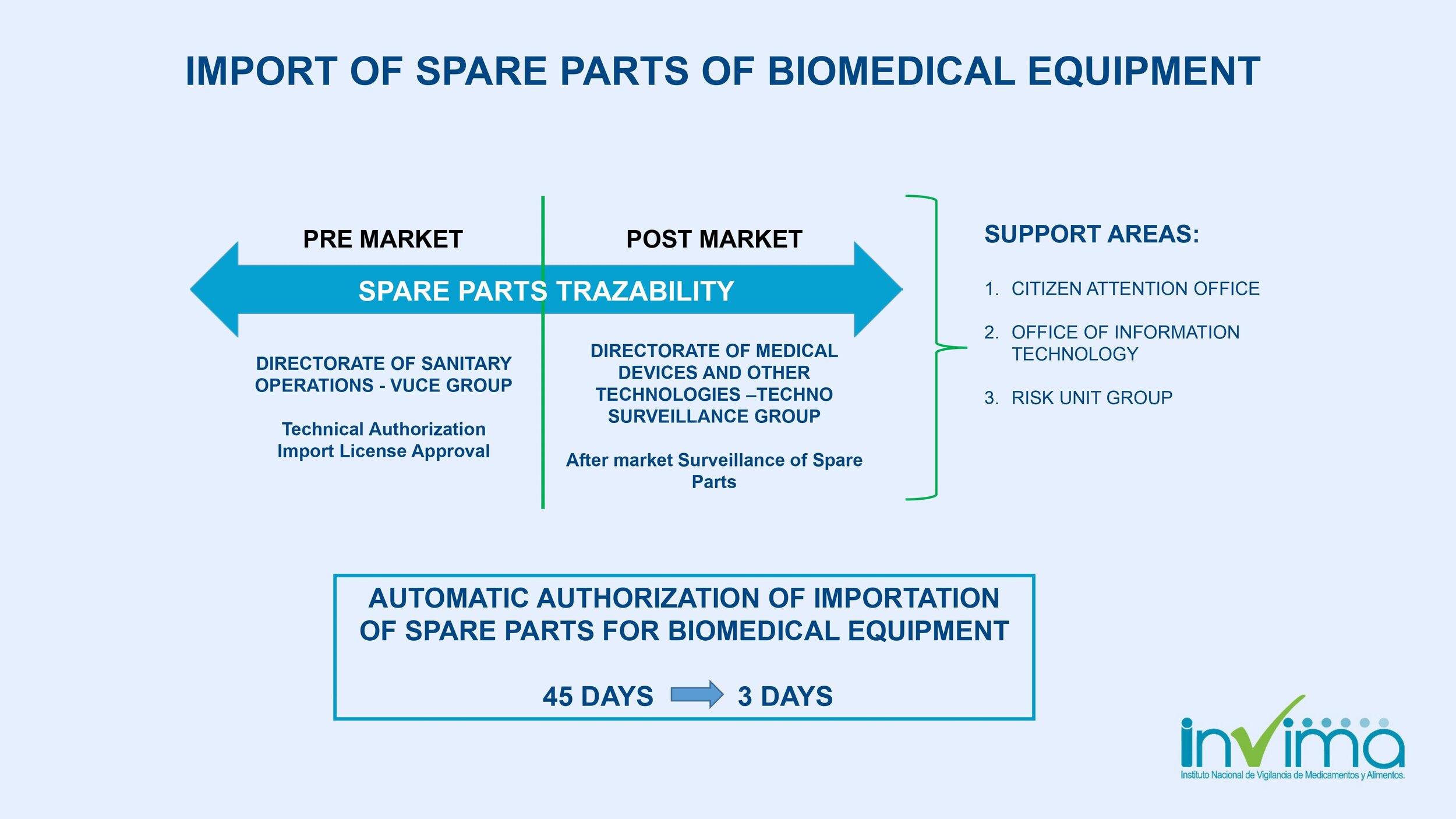




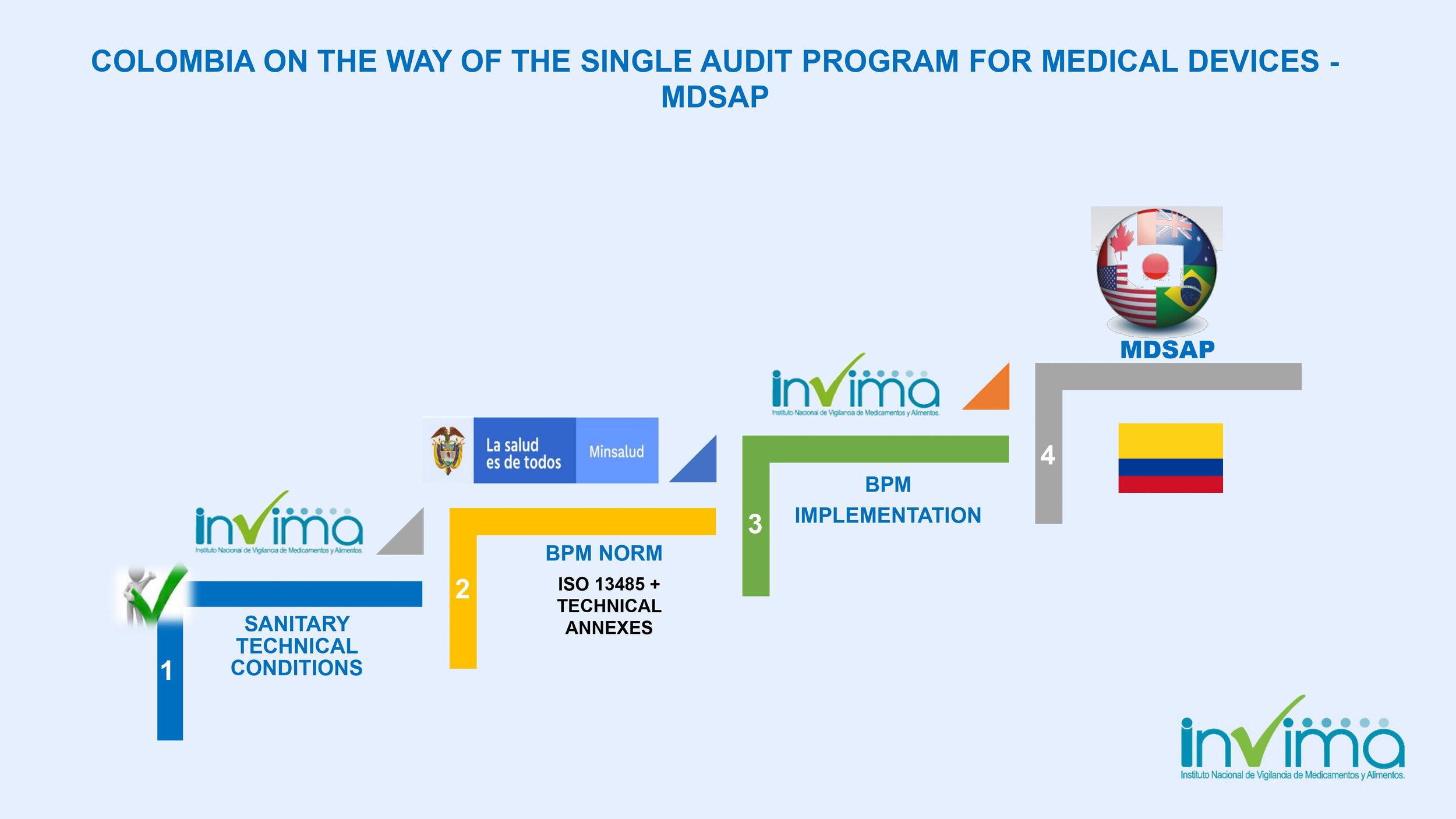

Is there a place I can find an overview of Colombia's regulatory framework for medical device and IVD registration?
Yes. Our blog here has an overview of Colombia's regulatory system. See below a summary of the regulatory registration process for medical devices and in vitro diagnostic (IVD) products.
What are the advantages of bioaccess™ representation as compared to working with a distributor in Colombia to obtain the necessary approvals necessary at INVIMA?
Very likely a distributor in Colombia has offered to put together a regulatory dossier package, pay the official INVIMA fee, and obtain market clearance for your medical device at INVIMA in Colombia —at no cost to you.
On the surface, this sounds like a very appealing proposal; however, be aware that very likely the distributor will place its company name as the titleholder of the INVIMA registration certificate, and this will seriously limit your ability to switch distributor in case your relationship with that distributor deteriorates.
We suggest you follow the recommendations of the US Department of Commerce as referenced in the question above. If your company in the US, Europe, or elsewhere is the titleholder of the INVIMA registration certificate, you will be able to have total control over the regulatory and commercial aspects of your business in Colombia.
If you let a distributor in Colombia be the titleholder of your product's INVIMA registration certificate, you won't be able to easily switch distributors/importers of record in case your relationship with that distributor deteriorates due to poor sales or other reasons. If your relationship with your initial distributor deteriorates, it will not be interested in transferring the INVIMA registration certificate of your products to a competing distributor in Colombia; under this scenario, you will have to re-register your products at INVIMA.
The ideal model to register a medical device in Colombia and have total control over your sales in the country is by having your product's INVIMA certificate issued under your foreign company's name and have bioaccess™ as your legal representative at INVIMA. This way, you will have bioaccess™ as a trusted local partner for all matters related to maintaining control of your sales in the country.
Under this model, bioaccess™ will have the power of attorney to represent your interest in Colombia, submit your technical file to INVIMA to obtain market clearance on your behalf, help you comply with any post-marketing reporting requirements, answer all the questions you may have about regulatory and business matters, and name new distributors/importers of record and/or storage facilities in your INVIMA registration certificate.
What's the difference between a registration holder and a legal representative in Colombia?
A registration holder is a third party that you pay to hold the registration of a medical device in a country. This service is relevant to foreign manufacturers that want to be the titleholders of a registration certificate in a country and where local regulations prevent foreign entities from being titleholders. A registration holder will hold the title of the foreign manufacturer's registration certificate in exchange for an annual fee. In Colombia, local regulations allow a foreign entity to be the titleholder of an INVIMA registration certificate. So, technically, foreign entities don't need the services of a registration holder in Colombia.
A legal representative is a local entity in Colombia that has power of attorney from the foreign manufacturer to apply for an INVIMA registration certificate on behalf of the foreign manufacturer's entity. This local entity/legal representative in Colombia will also be listed at INVIMA as the local contact for all matters related to legal notices and technovigilance/post-marketing surveillance of the registered product. Read more.
Is there a sample application form and INVIMA registration certificate that you can show me?
Yes. Please see a completed application form here and a registration certificate here.
Is the INVIMA registration process difficult to get through as a foreign manufacturer?
Not really. Just get a local company to be your registered agent (aka legal representative or authorized representative), have it submit your dossier file on your behalf, and wait 90 days for an answer (approval or a request for clarification/additional documentation). If you have marketing authorization or a Certificate of Free Sale from a reference country (i.e., GHTF-founding member countries: US, EU, Canada, Australia, or Japan), you will have a straightforward and relatively simple process to get your medical device approved by INVIMA. Read more here.
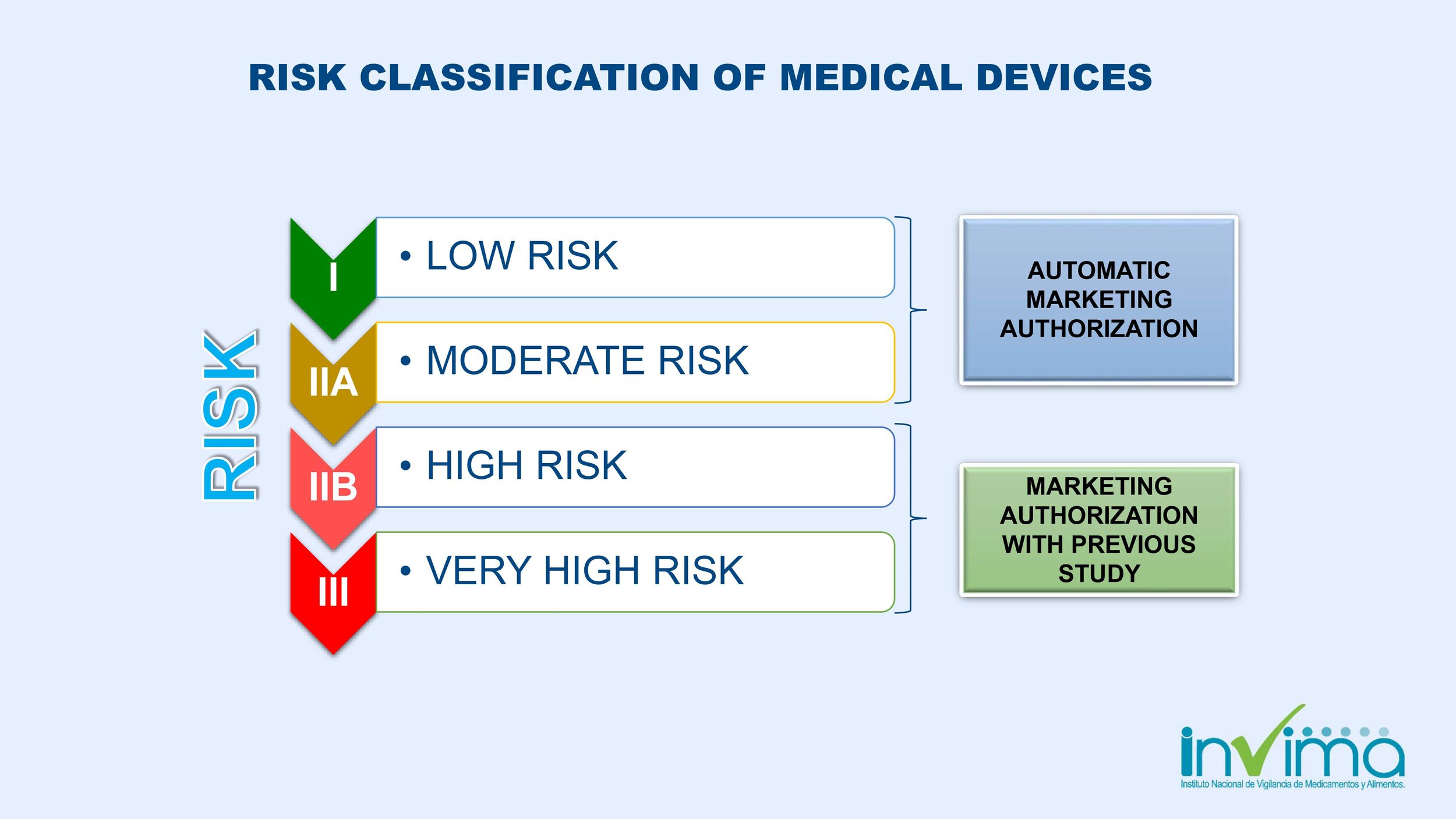
What is the medical device classification system in Colombia?
Colombia's INVIMA medical device classification system is based on the potential health risk related to the use and possible failure of a device. The criteria used involve the duration of contact with the body, how invasive the device is, and local effect vs systemic effect.
Class IIB
High risk
Subject to special controls in their design and manufacturing to show safety and efficacy
Example: Pulmonar ventilator, orthopedic implants
Class III
Very high risk
Subject to special controls to protect or maintain life
Made to prevent the deterioration of life
Their use represents a potential health risk or injury
Example: Heart valves, pacemakers
Class I
Low risk
Subject to general manufacturing controls
No potential harm of disease or injury
Not destined to protect or maintain life
Made to prevent the deterioration of human life
Examples: Surgical instruments, gauze
Class IIA
Moderate risk
Subject to special manufacturing controls to demonstrate their safety and efficacy
Example: Hypodermic needles, suction equipment
Source: ABC de Dispositivos Médicos (INVIMA)
Does INVIMA require that the manufacturing year of our device be the same as the year we plan to register the device in Colombia? In other words, if we plan to register our device in 2019, will it be mandatory to have a sticker on the bottom plate of the device stating the year of manufacturing as 2019? We manufactured our device last year (2018), and we are not going to manufacturer anything this yea (2019).
INVIMA's regulations do not require that manufacturers have a product manufacturing date to be the same as the year the product is being registered in Colombia. You can register your product in Colombia regardless of when your company manufactured the product. Read (in Spanish) page 44 of the INVIMA "ABC de Dispositivos Médicos" for more details.
Outside of ensuring we register and obtain marketing authorization under our company's name, is there any additional guidance you can provide for us on the best way to do it when working with each country within the Pacific Alliance?
Chile:
There are no mandated requirements for registering and obtaining marketing authorization for medical devices before they are sold in the national territory. However, this will likely change soon since there are discussions to pass a new law to change this and make registration mandatory.
We advise our clients to submit a quick and straight-forward voluntary registration so that you are grandfathered in when the new law is passed sometime soon.
The new law will align Chile’s registration requirements with the other countries of the Pacific Alliance (Colombia, Peru, Mexico) and make it mandatory to register and obtain marketing authorization for all medical devices before they are sold in the national territory.
Brazil, Mexico: I suggest you use the services of Mandala International (see here). We can make introductions upon request.
Independence from distributors:
Colombia allows foreign manufacturers to hold the title of the marketing authorization certificate under their names, and require foreign manufacturers to have a local authorized agent/legal representative with a power of attorney to receive notices and represent their interest at INVIMA.
All other countries require the titleholder to be a local entity so foreign manufacturers need the services of a local titleholder entity.
Do we need to engage the services of a third-party registration holder in Colombia?
No. Your foreign entity will be able to have its name listed as the owner of the INVIMA registration certificate in Colombia.
What's the timeline (after submission) for the registration of a medical device at INVIMA in Colombia?
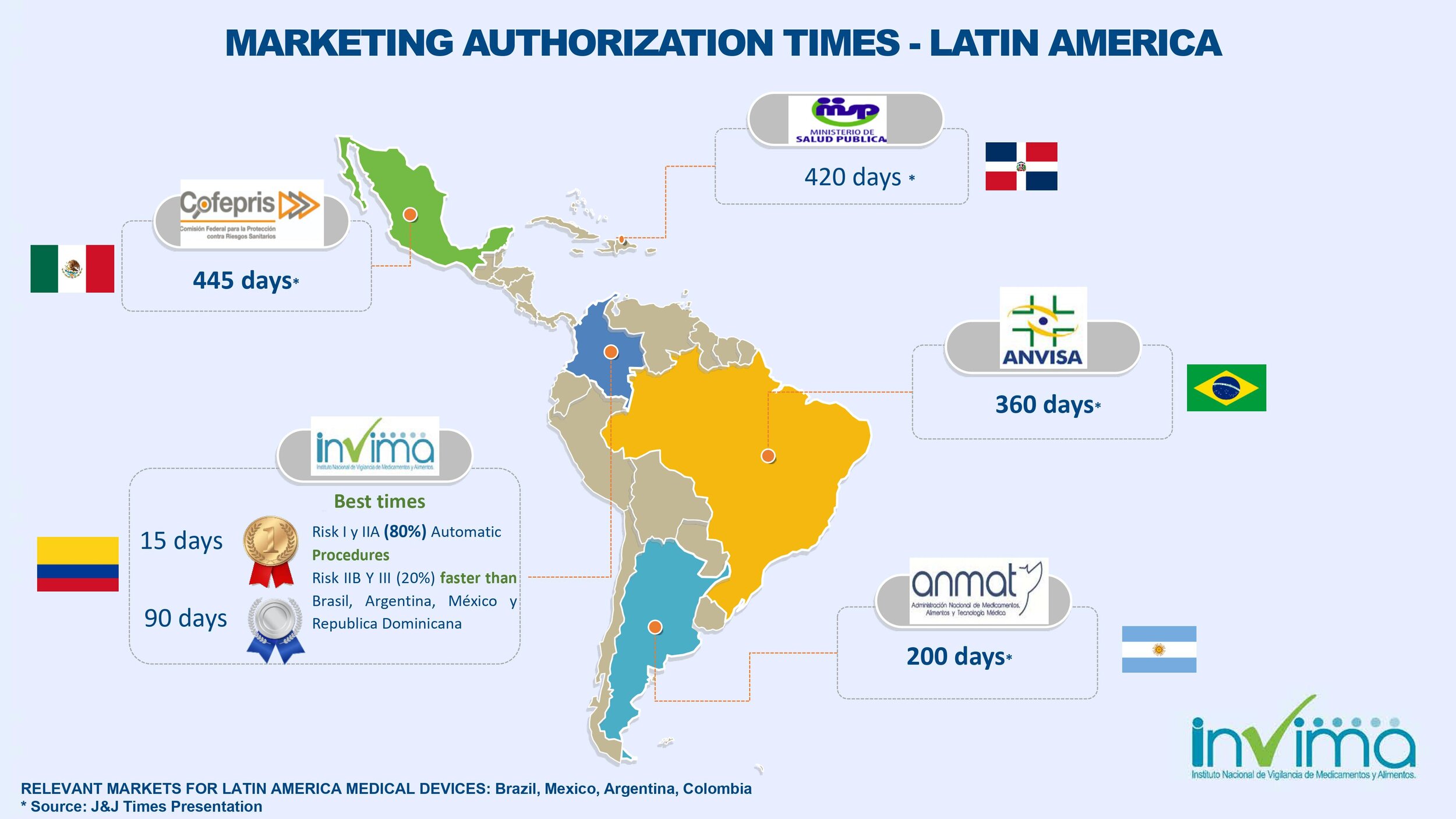
Colombia's INVIMA is the fastest regulatory approval agency for medical devices in Latin America. 15-90 business days (depending on your product classification). INVIMA must first review and approve Class IIb and III product applications prior to being placed onto the market, which on average takes 90 business days (approx.) depending on INVIMA’s current processing times. INVIMA automatically approves Class I and Class IIa products although it may take about 15 business days for INVIMA to issue the registration certificate.
Colombia's INVIMA is the fastest regulatory approval agency for medical devices in Latin America.
Can I group several medical device product references into one family (system or kit)?
A "system” or “kit are a group of medical devices that have the same indication for use, have the same manufacturer, and have organoleptic differences in size or features. INVIMA will allow grouping products in a “system” or “kit” if they comply with the following requirements:
For several medical device references to be included in one registration certificate, products must have:
Same risk classification
Same indication for use
Same generic denomination
Same registration titleholder
Same manufacturer
The devices may have differences in their material composition as long as that doesn't change their indication for use.
The composition of the different references is a variable that must be declared and supported by their biocompatibility and safety if applicable, but it doesn't limit the grouping at the time of submission of the registration application or at the time of trying to include a new reference into an existing registration certificate.
System: A set of products that each separately doesn't have a specific function and belong to the same manufacturer. The manufacturer must state that each reference is needed for the functioning of the set.
Kit: A group of products that could be sold separately with each having its own INVIMA registration but have a common purpose that makes them be sold as a “kit.” Each product in a “kit” may or may not require an INVIMA registration certificate under current regulations.
We already have a distributor in Colombia and they are registering our products. However, we still want our company to own the registration and be the titleholder of the INVIMA registration certificate so that we have total control and can add or remove importers/distributors if needed. Does INVIMA allow parallel registrations? Do we need to change the name of the products so as not to mix with the products that our distributor is registering in Colombia?
INVIMA allows parallel registrations in Colombia. You are free to register your products under your company's name without changing the name of the products.
We are looking to donate biomedical equipment to an institution in Colombia. Even though this is a one-time donation, do we need to register this product at Colombia's INVIMA?
Yes. INVIMA regulates the donations of biomedical equipment to a Colombian party. The equipment must have a valid INVIMA-issued registration certificate. Read more here (in Spanish).
Our medical product is manufactured in China. We need to get the Free Sale Certificate (FSC) and the ISO certificate "consularized" at the Colombian embassy/consulate in China. Should we send the original paper documents by courier (e.g., FedEx, DHL) to your address in Colombia? Can we send you scanned copies of the documents?
INVIMA will accept both documents (FSC, ISO cert.) in digital/scanned format.
INVIMA will accept all public documents from abroad in digital/scanned format with an apostille stamp or with a consularization stamp (at the Colombian embassy/consulate at the country of origin abroad). Consularization is the act of authenticating any legal document by the consul office of the document destination country (the consul signing and affixing a red ribbon to the document).
China is not a member of the Hague Apostille Convention. You cannot get a document with an apostille stamp in China. In China, you can only get your legal documents with a consularization stamp.
Costs
How much does it cost in USD to register my medical device at INVIMA in Colombia?
There are four main cost components you have to consider when planning to register your medical device at INVIMA in Colombia for marketing authorization:
INVIMA fees: They range anywhere between $850 and $1,000 depending on your device risk classification.
Translations fees: Expect to translate anywhere between 50 to 150 pages of documents and pay about $3.0 per page with a translator in Colombia.
bioaccess™ regulatory consulting fees: See fees here.
bioaccess™ registered agent/legal representation fees: See fees here.
What are the costs associated with translating the submission documents to Spanish?
The average standard rate in Colombia per page translated to Spanish is USD $3. Expect to translate anywhere between 50 to 150 pages of documents. We will ask you whether you want us to handle the translations locally in Colombia for you or if you prefer to send us the documents in Spanish. Please be aware that there are slight differences in the way Spanish-speaking countries in Latin America use the Spanish language. INVIMA prefers to receive documents in "Colombian" Spanish so that their staff and the general public can easily understand them in Colombia.
Distributors and Importers of Record (IOR)
How do I find a distributor in Colombia?
Finding a competent distributor is not easy. Foreign manufacturers need to conduct thorough due diligence before entering into business with a Colombian distribution partner and should be conservative in extending credit and be alert to payment delays. As one element in a prudent due diligence process, you should conduct background checks on potential Colombian distributors. Choosing the wrong distributor may cost you about three years in missed sales opportunities.
Follow this recommendations from the US Department of Commerce:
US manufacturers should consult a local attorney to execute an agency or distribution contract and to thoroughly vet the prospective partner by conducting a background check.
To secure an agent, representative, or distributor the foreign company must execute a contract that meets the provisions of the Colombian Commercial Code. This contract must be registered with the chamber of commerce where the agent/representative is located.
US manufacturers should be aware, however, that their ability to compete in Colombia could be hampered by unfair business practices such as contraband, counterfeiting, intellectual property rights violations, under-invoicing, money laundering, and dumping.
Source: Export.gov
It is important to choose a reputable distributor when entering the Colombian market, as the wrong one can lead to missed sales opportunities, poor relationships with end users and a damaged brand name. Working with local experts ensures that distributors are chosen objectively and evaluated with your specific business needs in mind. Read how we can help you create a market access plan and find a distributor in Colombia here.
Should I start the registration of my medical device at INVIMA in Colombia before I find a distributor or should I find a distributor first and then start the registration process?
The first question distributors usually ask is whether the manufacturer's products have regulatory registration or not. Having the registration (or at least starting the process before contacting potential distributors),
Signals the distributor that you are professional and serious in how you intend to enter the market.
Signals the distributor that they can start selling and making money faster (they don't have to wait for a registration that may take 3-6 months).
Signals the distributor that he won't be wasting their time speaking with you on a product that may or may not get registration for missing documentation (e.g. incorrect certificate of free sale, quality or GMP certificate, etc.).
It will give you negotiating power since you'll hold the title of the registration and will remove the distributor as an importer of record if it doesn't perform as expected. The best practice is that the manufacturer registers its products independently from the distributor, and the manufacturer has full control over changes in the registration (e.g., remove a distributor for lack of performance, add a new distributor/importer, etc.).
Our purpose is to get a local distributor who will invest money to register, import our medical devices, and market them locally. We are looking for local involvement. We are interested to have the local distributors get our medical devices registered at INVIMA.Is this the right strategy?
Colombia is the third largest medical technology market in Latin America (read more in our blog here).
The validity of an INVIMA registration in Colombia is 10 years and foreign companies can hold the registration under the name of their company overseas. This makes foreign manufacturers to prefer registering their products under their name and not let the distributor do it in Colombia.
If you let a distributor register your products under its name, it will have exclusivity to sell your products in Colombia for 10 years, and since you don’t own not control the registration of your products, you won’t be able to switch distributors at will.
How many importers of record (IOR) can we authorize? When shall we supply this information to you at bioaccess™? if we plan to have 2 or 3 distributors in Colombia and authorize all of them as the IOR, is this workable?
You can only name one (1) IOR in your INVIMA registration certificate. You must give us the information of this IOR right before we file your regulatory dossier at INVIMA. We will work locally in Colombia with your IOR to gather the documentation we need (i.e., certificate of incorporation, letter accepting their appointment as the IOR, their valid INVIMA-issued CCAA (Certificado de Capacidad de Almacenamiento y Acondicionamiento in Spanish).
How many distributors can we appoint per registration in Colombia? Example in Mexico we can have up to 5 distributors per registration.
As many as you need. In Colombia you don't necessarily have to list your distributors in your INVIMA registration certificate. You must name an importer of record (IOR) (which is usually a distributor). The company that you name as your IOR in Colombia must have a valid INVIMA-issued CCAA (Certificado de Capacidad de Almacenamiento y Acondicionamiento). If you only plan to have one distributor in Colombia, then most likely you will name this distributor as your IOR.
Our plan is to have 2 or 3 distributors in Colombia. What should we do?
Just name them as different importer of record (IOR).
Should I appoint my distributor as my regulatory registered agent/legal representative in Colombia?
Some manufacturers have considered appointing a Colombian distributor to fulfill this role. While it is possible to appoint a local distributor, there are valid reasons not to appoint a distributor as your registered agent/legal representative, including:
You may need to provide access to your device information and documentation (mandatory for registration purposes) and most companies prefer not to put (confidential) design information in the hands of their distributors.
A conflict of interest may occur in the event of recall and/or incident reporting between you (the manufacturer) and the distributor. If INVIMA questions an incident or a non-compliance that occurred in the distribution system, will your distributor defend his company or yours?
The distributor is focused on sales and marketing, not on regulatory affairs. They may not keep you up-to-date on regulatory changes in the market and provide timely warnings when changes affect your devices.
You may want to assign an initial distributor to be your importer of record (IOR) in Colombia, and later you may need to switch the name of your IOR in your INVIMA registration certificate. If you select an independent third-party as your registered agent/legal representative in Colombia, it will fully cooperate with you so that you control all regulatory aspects of your medical device on the market.
Should I register my medical device or let my distributor do it (and pay for it) in Colombia?
You should register your medical device under your company's name and pay for the INVIMA registration process. This way, you will own the registration and have total control over it. This is what the US Department of Commerce has to say about it:
"It is strongly recommended that U.S. companies process the registration under their name and not under the local distributor's name or else the U.S. company will not be able to change or add distributors during the lifetime of the registration, which is 10 years." —U.S. Commercial Service
Don't be tempted to let your distributor take control of the INVIMA registration process and pay for it. You will likely regret it later.
Are there any INVIMA requirements for distributors so that they can officially sell my company's products in Colombia? Does INVIMA and/or the regulatory registered agent/legal representative or manufacturer have to officially “recognize" them as commercial distributors?
No. However, if you appoint a local distributor in Colombia as your importer of record (IOR) in your INVIMA registration certificate, this distributor must be certified by INVIMA to store medical devices. In other words, this distributor must have a valid INVIMA-issued CCAA certificate.
Can my company register the products that a former distributor had already registered?
Yes. Colombian allows duplicate registrations. A foreign manufacturer can register its products in Colombia despite the fact that its current distributor may have already registered them. Bear in mind that your company will have to pay for this registration process as a new submission and re-submit all necessary documents (INVIMA will not grandfather a prior submission or give you access to prior documents).
bioaccess™, as your company's local regulatory registered agent/legal representative, could handle this process for you. Your company will have to name a new IOR on the new application submission.
What should I do if my company is the titleholder of an INVIMA registration certificate and wants to switch importer/distributor in Colombia?
If you are the titleholder of the INVIMA registration certificate, then it's a straightforward process to add or remove importers/distributors and will take about 30 days. If your prior distributor is named in your INVIMA registration certificate as the importer of record (IOR), then we can submit a certificate amendment request to INVIMA on your behalf.
You will need the following,
INVIMA form ASS-RSA-FM007 completed and signed by the certificate titleholder's legal representative or its attorney in fact in Colombia.
The Certificado de capacidad de almacenamiento y acondicionamiento (CCAA certificate) of your new importer/distributor.
The Certificado de cámara de comercio (incorporation certificate) of your new importer/distributor.
We are planning to change our distributor in Colombia. Our current distributor has ownership of the INVIMA registration certificate. Is it possible to transfer the registration to our (company) name? And if so, what will it take to do so?
The short answer is "yes" providing that your local distributor is on friendly terms with your company and is willing to sign the necessary documents to authorize the transfer of the INVIMA registration to your company's name.
If your distributor is the titleholder of the INVIMA registration certificate, you won't be able to process any changes to the INVIMA registration certificate without your distributor/titleholder cooperation and authorization.
There are two possible scenarios,
The distributor/titleholder is willing to sign the necessary documents to process the transfer of the INVIMA registration certificate from its name to your company's name:
Document requirements:
A completed INVIMA form (ASS-RSA-FM007) signed by the legal representative of the current titleholder (or its attorney in law).
A letter from the existing titleholder authorizing the amendment of the registration certificate.
The incorporation certificate of the new titleholder.
Time: It should take about 30 days or so depending on INVIMA’s processing time at the time of submission.
Cost:
The INVIMA filing fee is about $150 USD.
The distributor is not willing to sign the documents nor transfer the registration to your company's name: You will have to start the process from scratch, re-register your medical device and obtain market clearance at INVIMA (under your company's name).
Our old distributor doesn't want to transfer the INVIMA registration certificate that it got for our products. Will INVIMA permit two titleholders for the same product? Can we proceed with an application/submission for a new INVIMA registration certificate rather than filing for a change to the current certificate? Some countries permit this, others do not.
Yes, you can.
Can we transfer a medical device registration license or certificate without acceptance from the current license holder?
No, you can't.
There is an INVIMA registration certificate under a manufacturer's name (the manufacturer is the titleholder). I would like to amend this certificate so that it now lists a different manufacturer as its titleholder. Do I need the current importer of record/distributor's to approve this amendment?
No, you don't need the importer of record/distributor approval. What you need to do is submit an application to INVIMA to amend the registration certificate.
These are the document requirements, timeline, and costs:
A completed INVIMA form (ASS-RSA-FM007) signed by the legal representative of the current titleholder (or its attorney in law).
A letter from the existing titleholder authorizing the amendment of the registration certificate.
The incorporation certificate of the new titleholder.
Time: It should take about 30 days or so depending on INVIMA’s processing time at the time of submission.
Cost:
The INVIMA filing fee is about $150 USD.
Some manufacturers engage local key opinion leaders (KOLs) to promote their products. Can bioaccess™ help us?
Yes, this is part of having a hybrid approach to develop a market. This is an ongoing effort. We can become your country manager and manage engagement and interactions with KOLs. This will add an extra recurring monthly cost to your market access initiative. A hybrid approach requires that you have a thorough understanding of the local market and actively perform market development activities with your chosen distributor. Our blog has a couple of articles that talk about effective market access strategies.
Most companies fail because they have a hands-off approach (as opposed to hybrid) where they dump products to a distributor and hope that magically the distributor develops the market and sells it. Manufacturers that have a hand-off approach are usually not willing to invest in market development activities. Manufacturers that have a hybrid approach usually have someone who travels extensively to a country or a local country manager representing their interest, doing continuous market development, and advancing their market access plan.
A well thought out market access plan involves engaging KOLs (and compensating them for their services), putting together real-world data studies at insurance companies (they owned the patients in Colombia, not the hospitals), presenting the results at scientific conferences, engaging physicians who can prescribe the manufacturer’s product, organize marketing activities that guarantee top-of-mind, etc. These activities generate demand for your product so that distributors can fulfill it and provide training and post-sales support.
Market development has an investment and is not something that a distributor is usually willing to do. Distributors are usually smaller under-financed companies that get involved with products that can give them a quick sale, and they are willing to quickly forget about products that don’t. I suggest you read some of the articles we’ve written on our blog about this topic.
bioaccess™ importer of record (IOR) service allows foreign medical device and IVD companies to expand into Colombia with ease. Our IOR service simplifies the market clearance of your medical product at INVIMA and saves you from the costs of establishing a legal entity or from waiting to find a suitable distributor.
Submission
How long does it take to register and obtain regulatory registration/marketing approval/clearance for a medical device in Colombia?
Assembly and translations of dossier file: Approx. 30-45 days after receipt of all your technical and legal documents.
Application and dossier file submission, follow up, and approval of registration by INVIMA:
Medical devices: Class IIb and III device applications must first be reviewed and approved by INVIMA prior to being placed onto the market, which on average takes 90 business days.
INVIMA automatically approves Class I and Class IIa devices but it may take about 15 business days to issue the registration certificate.
How do I register my medical device at INVIMA in Colombia?
These are the nine (9) steps to register your medical device at INVIMA in Colombia:
FREE DOWNLOAD
Learn how to register your medical device and obtain marketing authorization in colombia
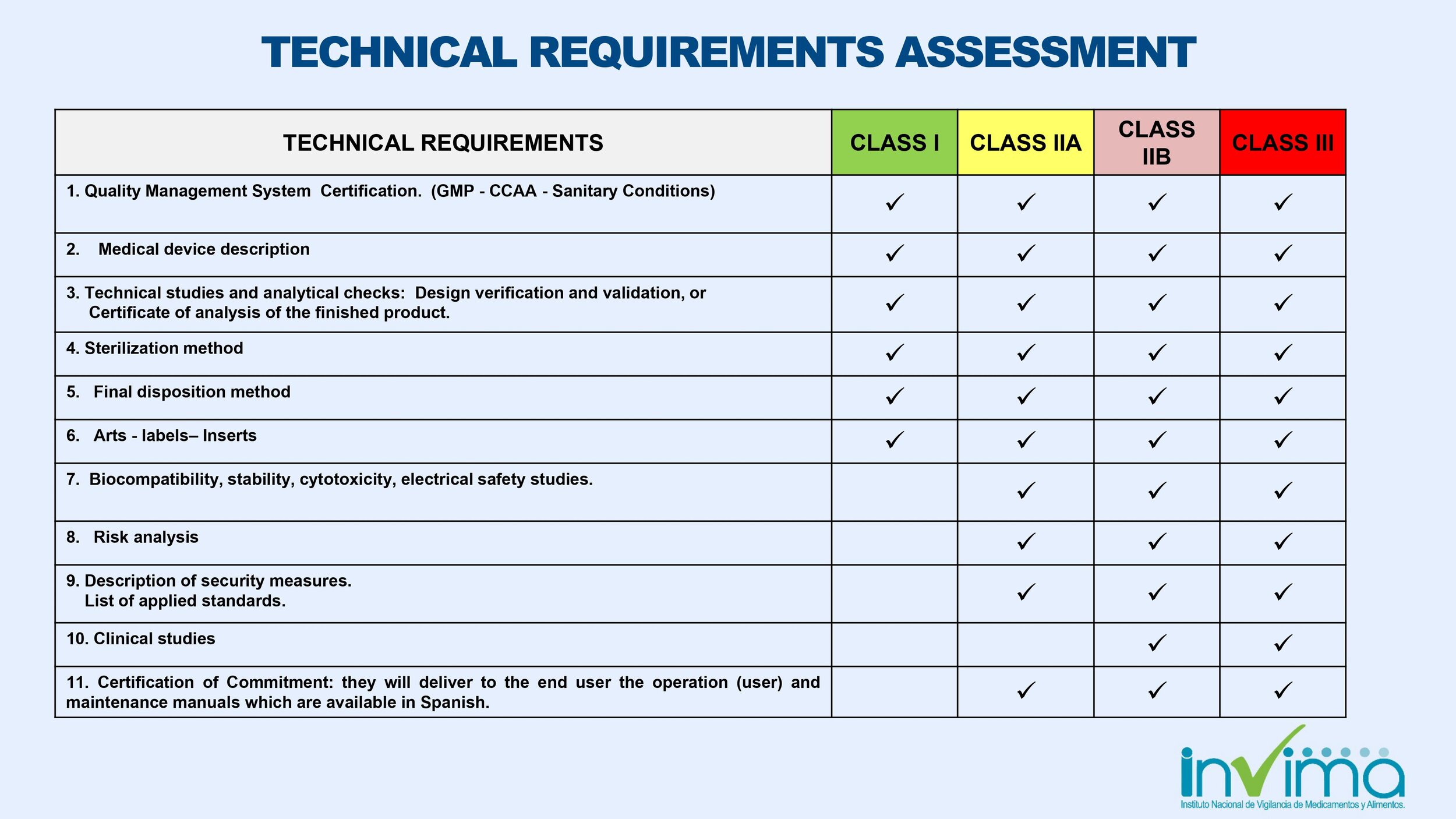
Determine the correct classification of your medical device. Device classification in Colombia follows a four-tiered risk model (Class I, Class IIa, Class IIb and Class III) that is similar to the classification scheme used in Europe.
If you have no local presence in Colombia, then you must appoint an in-country representative (aka authorized/registered agent or legal representative). If you appoint your distributor, it may list itself as the owner of the INVIMA registration, which is 10 years (not good!). If you appoint an uninterested third-party as your legal representative, it will request a power of attorney from you to manage your registration process, but your foreign company will retain ownership (very good!).
Appoint an importer of record (IOR) with a valid INVIMA-issued certification to storage medical devices; this certificate is called a “CCAA certificate.” bioaccess™ can become your temporary IOR while you find a permanent IOR/distributor in Colombia. Read more.
Obtain a Certificate of Free Sale (CFS) or Certificate to Foreign Government (CFG) from your home country or from a GHTF-founding member country (i.e., Australia, Canada, European Union, Japan, and the United States of America). If your medical device is not sold in the country where it is manufactured, you must include a certificate issued by the competent authority in the manufacturing country that authorizes the production of the device, and proof of market clearance from one of the reference countries indicated above.
Provide quality system certificate, e.g. ISO 13485.
Provide product information and commercial history of the product; test reports will be required for Class IIa, IIb, and III devices, and clinical data will be required for Class IIb and Class III devices.
Your registered agent/legal representative will submit your application dossier to INVIMA in Spanish and will pay the required application fee.
Once approved, INVIMA will issue a registration certificate (aka “registro sanitario” in Spanish) valid for ten (10) years. By default, INVIMA automatically approves Class I and IIa applications, so you may begin importing and selling immediately after you receive your registration certificate in about 15 business days. However, INVIMA will still review the application and may request additional information. Once approved, INVIMA will issue your registration certificate. For Class IIb and Class III devices, INVIMA will take about 90 business days to review your registration application and may approve your application, ask follow-up questions, or request additional documentation. If all requirements are met, INVIMA will issue your certificate.
You may begin importing, marketing, and selling your device in Colombia. INVIMA registrations are valid for 10 years. Application renewals are due to INVIMA three (3) months before the expiration of your registration certificate.
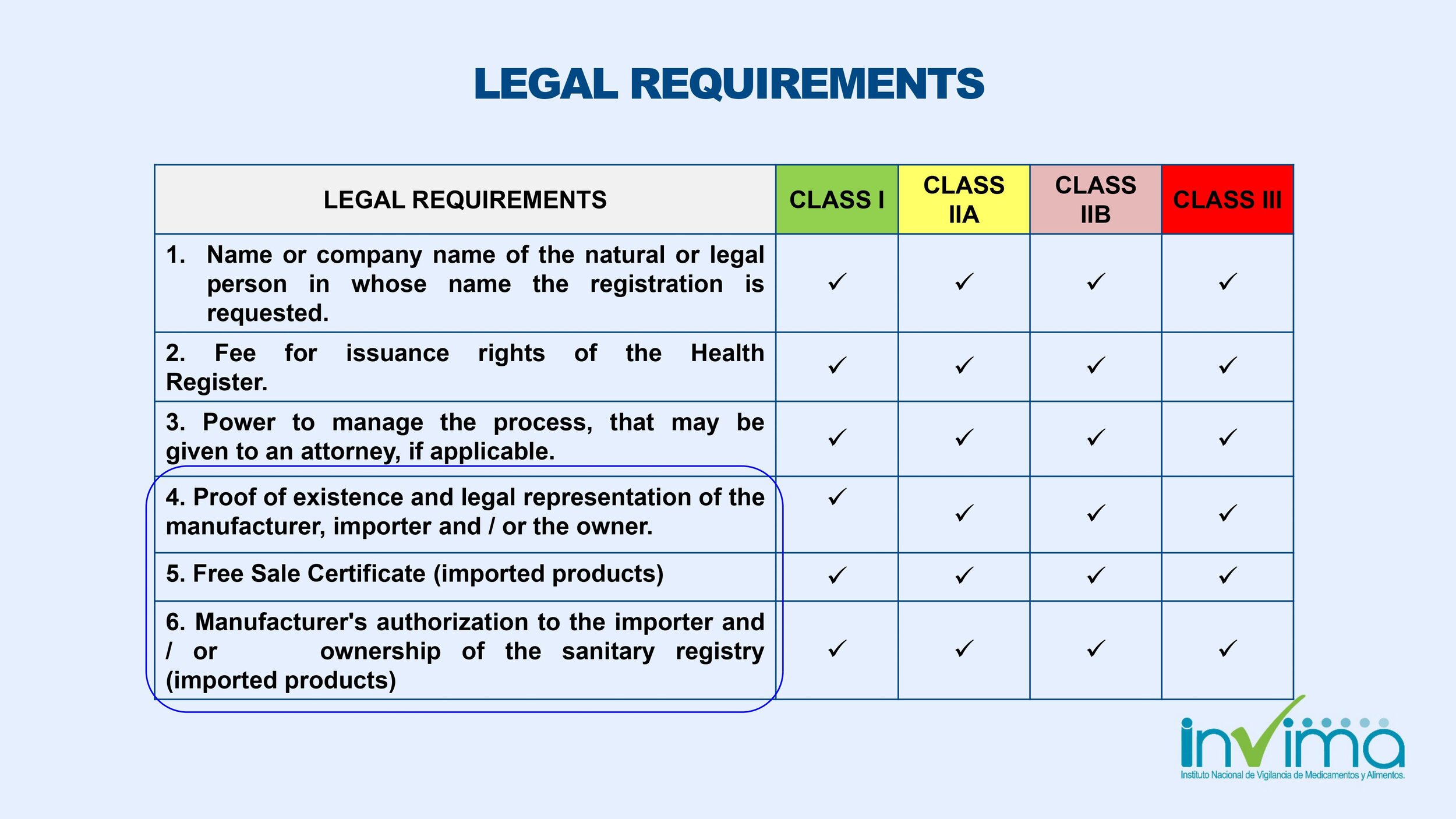
What are the document requirements to register a medical device at INVIMA in Colombia?
Certificate of Free Sale (CFS) or Certificate to Foreign Government (CFG) from your home country or an INVIMA recognized market (e.g., USA/FDA, EU/CE Marking, Australia, Canada, Japan). If your medical device is not sold in the country where it is manufactured, you must include a certificate issued by the competent authority in the manufacturing country that authorizes the production of the device, and proof of market clearance from one of the reference countries indicated above.
Quality system certificate (e.g., ISO 13485, GMP).
Product information and commercial history of the product; test reports will be required for Class IIa, IIb and III devices, and clinical data will be required for Class IIb and Class III devices.
Declaration of conformity (CoF) issued and signed by the manufacturer compliant with the international reference standards it meets, on which the name of the medical product becomes apparent, if not already on the certificate of free sale.
Completed INVIMA's application form
Technical and legal documentation (translated to Spanish)
Note: This is a simplified overview of the document requirements. Click here for a more detailed list of document requirements. INVIMA may choose to audit your submission and request more documents, which will add time to your approval.
Is apostille the documents the same as notarized?
No, they are two different acts taken on a document. An apostille is a form of authentication between different countries, in essence, is a certificate issued by the Department of Foreign Affairs verifying the genuineness of the signature and/or seal of a public officer, while a notarization is simply a way of identifying the identities of the parties signing a document.
Does the Certificate of Free Sale, the Certificate to Foreign Government (CFG), the ISO certificate, or any other government-issued or official document (e.g., notarized letter or power of attorney) need to have an apostille affixed to them before submission to INVIMA?
Yes. All foreign official and legal documents must have an apostille (or certificate of authentication) at the country of origin. In addition, they must be translated to Spanish by a Colombia-Ministry of Foreign Affairs-certified translator.
What parties do I have to name in my medical device INVIMA registration application in Colombia?
You will need to name three parties in your application to INVIMA:
The titleholder of the registration certificate (e.g., your company as a foreign manufacturer)
Manufacturer: It could be a local entity in Colombia or your foreign entity.
An importer of record (IOR) company (e.g., your local distributor(s)) with a valid INVIMA-issued storage certificate (aka CCAA certificate). bioaccess™ can become your temporary IOR while you find a permanent IOR/distributor in Colombia. Read more.
Storage facility (e.g., your local distributor(s)) with a valid INVIMA-issued storage certificate (aka CCAA certificate).
INVIMA issues a special certificate to medical device storage companies (aka CCAA or Certificado de Capacidad de Almacenamiento y/o Acondicionamiento). You as a foreign manufacturer (or a local third-party that you appoint) will be the titleholder of the registration. Any local company in Colombia could be your IOR as long as it is certified by INVIMA to store medical products with a valid CCAA certificate.
My company doesn't have FDA, CE Marking, or approval in Australia, Japan, or Canada. Can I still get market clearance for my medical device in Colombia?
Colombia’s regulatory agency (INVIMA) does not currently allow for the registration of medical devices that don’t have a certificate of free sale (CFS) from their country of manufacture or regulatory approval from a reference country (EU, USA, Canada, Japan, and Australia). There is a strong lobbying effort in Colombia to change the current regulation so that in cases like yours —a company that doesn’t have a (CFS or regulatory approval in a reference country)— can still get market clearance for their products in Colombia.
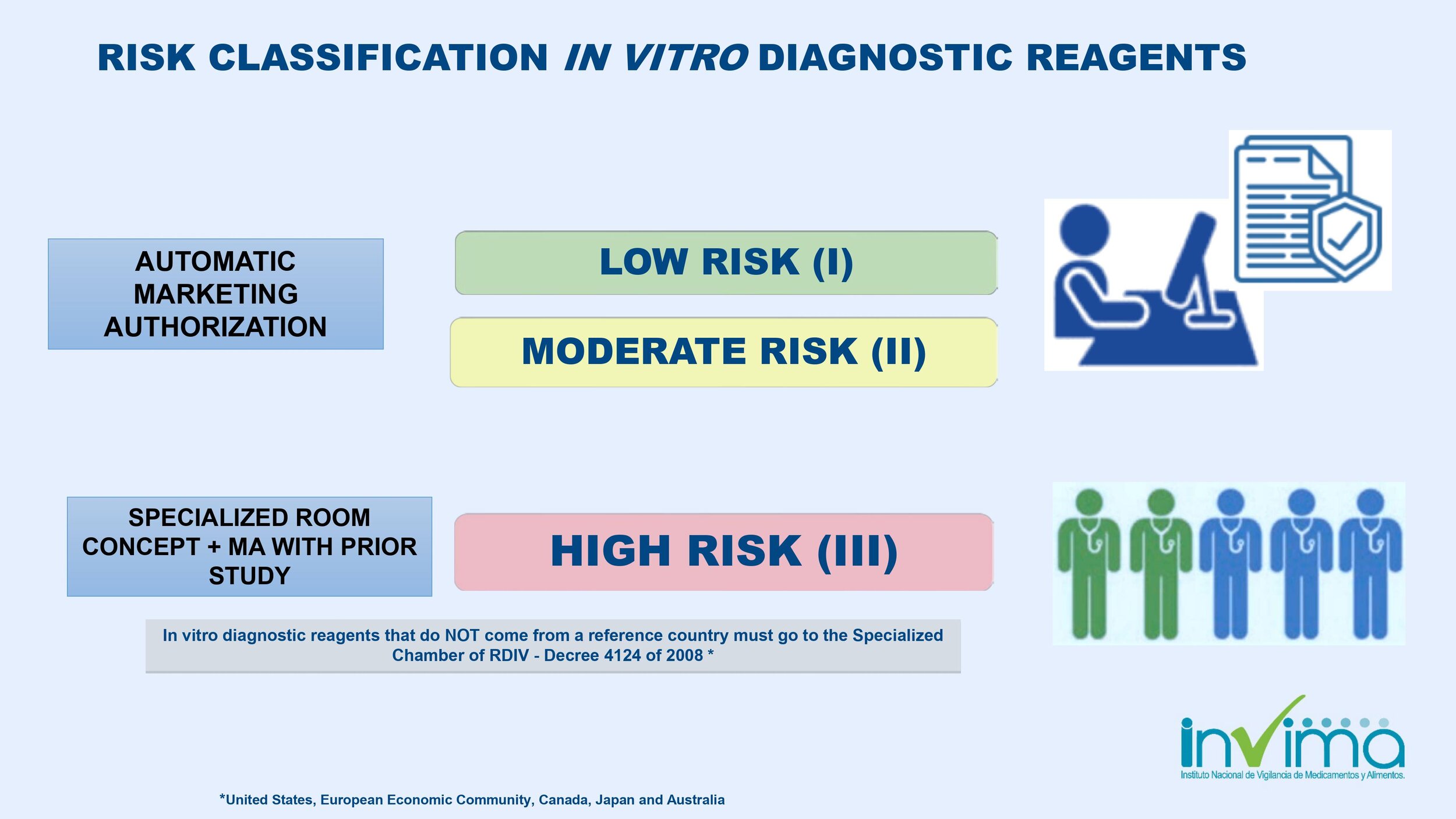
Is the list of reference countries limited to Canada, the US, EU, Japan, or Australia? Will registration in China be recognized? Will registration in Brazil and Mexico be recognized?
Yes, the list of reference countries only includes the US, EU, Japan, or Australia. China is not a reference country. If the manufacturer only has home country approval (e.g., China), then INVIMA will have to review the technical file in more detail, and it will take longer to get approval. There's no INVIMA recognition of registrations in Brazil or Mexico. The fastest way to get INVIMA registration approval is for manufacturers to have a free sale certificate from a reference country.
Should the registration holder be a local company in Colombia?
No. INVIMA allows foreign entities to be the title holders of their product registration. You must appoint a local legal representative to interact with INVIMA and receive notices, report adverse events, etc.
Does my company have to appoint a regulatory registered/legal/authorized representative/agent in Colombia?
Colombian law does not specifically mandate foreign medical product manufacturers to appoint an in-country regulatory registered agent/legal representative. Most foreign manufacturers that do not have a local office in Colombia appoint an in-country registered agent/legal representative for practical reasons (e.g., to manage the submission of the initial registration, receipt of notices, reporting of adverse events, to file the mandated annual post-market surveillance report, and to be the liaison between the manufacturer and its distributors).
What is the role of a regulatory registered/legal/authorized representative/agent in Colombia?
Foreign manufacturers must issue the power of attorney to a local third party in Colombia if they would like to have that third party represent their interest while doing business in Colombia and communicating with INVIMA on their behalf.
Most foreign manufacturers that have more than one distributor in Colombia, appoint a registered agent/legal representative to submit their INVIMA registration application, and to manage all regulatory aspects of doing business in Colombia. This way, foreign manufacturers have an independent third party looking after their interest in Colombia.
The registered agent/legal representative acts as a liaison between you, INVIMA, and any other private or government entity. Medical device manufacturers that do not have a physical location within Colombia usually appoint and maintain that registered agent/legal representative for as long as they market and sell their products in Colombia.
Your registered agent/legal representative in Colombia will manage your product registration process, will interact with INVIMA on your behalf, will receive legal notices on your behalf, and will ensure that you comply with local post-marketing surveillance requirements (i.e., filing the mandatory annual report).
bioaccess™ can act as your registered agent/legal representative in Colombia, fulfilling all regulatory responsibilities per national health regulations. When you assign power of attorney to us,
We will place our name, mailing address, email address, and phone number on your device submissions and registrations to INVIMA.
We will receive all INVIMA notices about your product, review them, and share them with you to establish the best course of action.
Upon request by INVIMA, we will provide information about your product, and the names and addresses of distributors established in Colombia. We will have access to your product documentation for inspection by INVIMA if requested. Note that we can only supply this information for inspection by INVIMA upon written permission from you and cannot share it with any other person or entity.
We will be available to make changes (e.g., switching importer of record) to your existing registration certificate.
We will be available to coordinate inquiries, analysis, and responses for reportable adverse events as reported by users, distributors, importers of record, or INVIMA. We will help you determine the best response.
We will be available to provide technical assistance for the reporting of adverse events as reported by users, distributors, and importer of record. bioaccess™ can become your temporary IOR while you find a permanent IOR/distributor in Colombia. Read more.
We will be available to make certain your distributor or importer of record (IOR) follows Colombia's post-market surveillance regulations.
We will be available to act as a liaison between INVIMA and you in case of recall or risk for product withdrawal from the market.
We will be available to submit to INVIMA the required annual report (mandated for high-risk/controlled technology medical devices) stating:
Number of imported products
Number of sold products
Location of all sold products
The serial number of all sold products
Serious adverse events and the actions taken
What's the role of an importer of record (IOR) in Colombia?
You will need to name the following parties in your medical device registration application to INVIMA:
The titleholder of the registration certificate (e.g., your company as a foreign manufacturer).
The foreign manufacturer.
An importer of record (IOR) company (e.g., your local distributor(s)) with a valid INVIMA-issued storage certificate (aka CCAA certificate).
A local storage facility with a valid CCAA certificate.
INVIMA issues a special certificate to medical device storage companies (aka CCAA or Certificado de Capacidad de Almacenamiento y/o Acondicionamiento). You as a foreign manufacturer (or a local third-party that you appoint) will be the titleholder of the registration. An importer of record (IOR) is a legally formed entity under Colombian law that has been classified as an "importer" by Colombia's custom agency (DIAN). Any IOR in Colombia that is certified by INVIMA to store medical products could be your importer and storage company.
If a manufacturer only has one distributor in Colombia, very likely it will appoint this distributor as its IOR. If a manufacturer has more than one distributor in Colombia, it will very likely appoint a third-party company as its IOR (preferably a logistics company that is also certified by INVIMA as a storage company).
The role of the IOR is to apply for import permits at Colombia's Ministry of Industry and Commerce (VUCE office) and to interact with Colombia's customs agency (DIAN) to nationalize the manufacturer's medical devices in Colombia.
What are the specific labeling requirements for submission to INVIMA?
Original artwork (if applicable).
The artwork in Spanish with at least the following information: product name or reference, manufacturer's name, and address, and internationally recognized safety symbols.
Artwork for a separate label in the form of a sticker with the following information: Name of the importer of record, product name, product model or reference number, importer of record's name and address, registration certificate number, and classification of the device (I, IIA, IIB y III).
Which documents must be in Spanish and which ones are accepted in English? What documents does my company need to translate?
The number of documents to translate will depend on the classification of your device. The steps above tell you what documents you generally need to submit to INVIMA in Spanish. Registration materials pertaining to biocompatibility, risk analysis, sterilization, and clinical studies and test reports may now be submitted to reviewers in their language of origin; summaries of study descriptions, methods, and conclusions must be provided in Spanish.
Once we review your product information, we will be able to tell you exactly what documents you need for submission. Please bear in mind that after submission and INVIMA internal technical review, it may ask for additional documents at its discretion. You must translate to Spanish your devices' operations and maintenance manuals. Please review INVIMA's official website (in Spanish) for detailed information about submission requirements.
Any legal document (e.g. power of attorney) must have an apostille and must have its respective "official" translation by a translator who is certified by Colombia's Ministry of Foreign Affairs.
How long will it take for bioaccess™ to review our documents and assemble the final dossier package to INVIMA?
Generally, we will take about 30-45 days. The time will depend on whether you have all the technical and legal documents ready for us to review or not. You will need to notarize and apostille some legal documents and ship them to us by courier; this may delay the review and assembly process.
Can my company start selling in Colombia as soon as INVIMA receives our application submission for medical device registration?
INVIMA automatically approves Class I and IIa applications, so you can begin selling right away (INVIMA will take about 15 business days to issue your registration certificate). The agency will still review the application, and manufacturers must respond to any additional information requests within 30 days. Failure to comply will result in approval being revoked. For Class IIb and Class III, INVIMA must review and approve your application before you can begin selling; the review could take up to 90 business days. During this review, INVIMA may ask follow-up questions or request additional information.
What is the description of the automatic approval for low-risk (Class I, IIa) at INVIMA?
INVIMA does indeed review and approve the dossier for low-risk (class I, IIa) medical devices. This is clearly stated in Decree 4725 of 2005 (see here).
Article 17 of Decree 4725 of 2005 states that INVIMA will issue automatic approval for low-risk medical devices (class I, IIa) after complying with the legal and technical requirements stated in the same Decree.
Article 20 of the same Decree defines the minimum legal and technical requirements for the automatic approval of low-risk medical devices.
Article 22 of the same Decree defines the procedure to obtain automatic registration of low-risk medical devices at INVIMA. This procedure describes how to submit the dossier to include completing an INVIMA form and attaching the legal and technical documents required in Article 20 of the same Decree.
Article 22 also states that upon submission, INVIMA will immediately review the dossier for low-risk devices to make sure they are complete. If they are complete after the INVIMA review, then INVIMA will issue the registration certificate within two (2) days. If the dossier is incomplete, then INVIMA will reject the submission.
Article 22 also states that after INVIMA issues an automatic registration certificate for a low-risk medical device, INVIMA can verify the original documentation. If during this verification process, INVIMA discovers that something is missing in the dossier, then INVIMA will ask the applicant for the missing documentation/information and will give it up to ninety (90) days to respond and provide the missing documentation/information. If the applicant doesn't provide the missing documentation/information within the 90 days, then INVIMA will suspend the registration certificate, and then within 90 days, INVIMA will cancel the registration certificate.
Is home country approval required to register my medical device in Colombia?
For lower-risk devices, INVIMA has reduced some registration requirements; INVIMA now accepts Certificates of Free Sale (CFS) from GHTF-founding member countries (i.e., Australia, Canada, European Union, Japan, and the United States) in place of certain required documents.
Home-country approval (or approval in a GHTF-founding member country) is a prerequisite for market authorization in Colombia. Foreign manufacturers have the option to maintain full control over their registrations in the Colombian medical device market. This means that you are allowed to place your company name as the titleholder of the INVIMA registration certificate.
What if we do not have ISO 13485 certification for medical devices?
The regulation requires proof of a QMS (such as ISO 13485); however, if your company doesn't have it, then you can meet this requirement by providing an ISO 9001 certificate, an FDA Establishment Report, or some other equivalent document.
How do I control my approval with bioaccess™ as my registered agent/legal representative?
When foreign manufacturers trust their local distributor to register their products in Colombia —instead of an independent third-party regulatory registered agent/legal representative— the distributor will often list itself as both the holder and importer of record (IOR) of the registration. This means that the distributor is in total control —for 10 years— of your product registration (and commercialization) in Colombia; any future modifications, such as adding a new distributor, models, etc., would need to be authorized and processed at INVIMA by the distributor itself (not good!).
Please note that it's not in the distributor's best interest to relinquish its ownership of the registration certificate. This forces the foreign manufacturer to re-start the process of registering the product when in need to change its distributor in Colombia —duplicate product registrations are allowed in Colombia.
As your registered agent/legal representative, bioaccess™ will register your product in your foreign company's name, ensuring that you have 100% control over your registration and the commercialization of your products; this way, you will be able to switch at will the importer of record listed in your INVIMA medical device registration.
Whom do I name as an importer of record (IOR) in my product registration certificate application submission to INVIMA?
On the application form for the new registration, we will add the name of the importer of record (IOR) of your preference. In most cases, the importer of record is a distributor with a valid INVIMA-issued CCAA certificate.
Does INVIMA need the name of my company's importer of record (IOR) in Colombia before the product registration certificate application is submitted to INVIMA?
Yes. INVIMA's medical device registration application form requires the name of your IOR upon submission. INVIMA has a rigorous certification process (also known as Certificado de Capacidad de Almacenamiento y Acondicionamiento or CCAA) to make sure importers of medical devices comply with storage, quality, and personnel regulations (Resolution 4002 of 2007). Any local entity in Colombia that has the CCAA certificate can be your importer of record (IOR).
What power does the importer of record (IOR) have over my company's distributor(s) or regulatory registered agent/legal representative?
None. The IOR's only role is to be the official importer (and storage facility) of your company's medical devices in the Colombian territory. Colombia's customs agency (DIAN) will only let your company's IOR nationalize your products in the Colombian territory.
No other third party in Colombia will be able to import your company's products and sell them to end-users. Your company's regulatory registered agent/legal representative in Colombia will be able to act on your behalf to change the name of the IOR at your company's will.
Your company's IOR does not control your regulatory registered agent/legal representative nor it controls your distributors. Your company has full regulatory and commercial control over its IOR and its distributor(s).
CORONAVIRUS COVID-19
Does Colombia's INVIMA have a simplified fast-track emergency use authorization (EUA) process for medical products?
Yes. These are the simplified temporary fast-track EUA requirements:
Importer’s name and tax ID number.
Free sale certificate from the country of origin/manufacture or from a reference country (US, Canada, EU, Japan, Australia).
Quality certificate.
User’s manual and any other technical documentation that INVIMA deems appropriate for the product.
Label(s).
Proforma invoice of your planned product shipment to the importer in Colombia.
In what situation can we take advantage of the EUA in Colombia to sell my medical product?
INVIMA created the EUA program in cases where a local Colombian buyer needs to purchase COVID-19-related medical products to supply the local demand from a specific healthcare provider. If you, as a foreign manufacturer, have already identified a one-time opportunity to sell your product to a Colombian buyer, then you can use the EUA.
The EUA was not created to bypass the standard INVIMA regulatory approval pathways for medical products. The EUA was created to satisfy a specific demand for a COVID-19-related product where the product is not locally available in Colombia, and a buyer needs to quickly import it into the country.
What are the products with flexible requirements covered under the EUA at Colombia's INVIMA?
INVIMA has announced that the manufacture and import of the listed "unavailable essential medical devices and IVDs" are exempt from sanitary registration and will be temporarily authorized by INVIMA as long as they comply with requirements. Notably:
Non-sterile personal protection elements do not require sanitary registration or any type of approval by INVIMA.
Non-sterile protective suits for medical personnel do not require any paperwork with INVIMA.
Sterile protective suits are exempt from sanitary registration but subject to certain requirements to obtain an import or manufacturing authorization.
Hygiene products recommended for the prevention of COVID-19, with an alcohol concentration between 60% and 69%, require mandatory health notification.
Household hygiene products for disinfection, floor and surface cleaners, as well as dishwashers require mandatory health notification.
Disinfectant hygiene products used at the industrial, hospital market or for exclusive use in shopping malls and schools do not require mandatory health notification.
Does the importer of a medical product covered under the EUA need to have an INVIMA-issued valid CCAA certificate?
No. Any legal entity duly incorporated in Colombia can import medical products under the EUA as long as Colombia's custom and tax agency (DIAN) lists that entity as an importer in the entity's tax ID certificate.
What are the costs (USD) associated with obtaining an special import permit under the EUA at Colombia's INVIMA?
Official INVIMA fee for an import permit related to the nationalization of a medical product destined to be used during the COVID-19 pandemic: $50.
Dossier assembly and submission for expedited temporary approval to import and commercialize medical products for use during the COVID-19 pandemic in Colombia: $1,750 (bioaccess professional fee).
Translations.
What's the timeline to register a medical product under the EUA in Colombia?
One to two weeks after submission depending on INVIMA's processing time at the time of the application.
If an EUA is approved, would we receive a standard INVIMA registration certificate/license? Would any special conditions apply (in terms of duration, renewal, allowed use, etc.)? Would we need to provide additional documents?
No, you will not receive a standard INVIMA registration certificate/license. INVIMA will issue your buyer in Colombia will receive an “OK to import” temporary permit, and will list the specific quantities of your product that your buyer will import into Colombia.
It will just be a temporary authorization to your buyer in Colombia to import a specific number of products into Colombia. This will be a one-time temporary authorization to cover a specific need for a COVID-19-related product that is not available in the Colombian market. If your buyer in Colombia needs more of your products for another COVID-19-related sales opportunity, then it will need to apply for another EUA temporary permit.
Post-Submission
If I already registered my product in Colombia, will I be able to sell it in other Latin American countries?
No. You will have to register your product in each individual country. The countries that are part of the Pacific Alliance —Chile, Colombia, Ecuador, Mexico, and Peru— are advancing a Regulatory Coherence and Medical Device Regulatory Convergence Initiative. These efforts are augmented by the work of the APEC Life Sciences Innovation Forum (LSIF), Regulatory Harmonization Steering Committee (RHSC) which includes Chile, Mexico, and Peru. The RHSC promotes a coordinated approach to regulatory harmonization and capacity-building efforts across the region. The goal of this initiative is to have a common regulatory market with the Pacific Alliance countries so that if you register your product in one Alliance-member country, its regulatory approval will be recognized by the other member countries. This initiative has not been finalized yet.
How can I know that my product has been registered with INVIMA in Colombia?
You can go to INVIMA's website and search (in Spanish) for “registro sanitario” (sanitary registration). You will be able to select your type of product and search using several keyword options.
Can I transfer my existing INVIMA registration certificate to another registration holder?
Registrations can be transferred in Colombia as long as the original holder (a local distributor, in most cases) would agree. If the holder is a distributor, it is usually not willing to give up control of registration, and often foreign medical device companies need to submit a new application and incur additional costs. When you appoint bioaccess™ as your regulatory registered agent/legal representative, you can change your registration as needed and have total control over the commercialization of your product in Colombia. bioaccess™ will submit the INVIMA application with your foreign company as the holder and controller of the registration.
What would be the purpose or reasons for which our medical product would need to be stored at an INVIMA-certified facility?
Colombia's regulations require that medical technologies (medical devices and in-vitro diagnostics products) be imported and stored by a local entity that has a valid INVIMA certification to store such products. This is called the Certificado de Capacidad de Almacenamiento y Acondicionamiento (CCAA) certification and is mandatory for all medical technologies in the country. So, your importer of record (IOR) in Colombia must have this CCAA certificate to legally import, handle, and store your products in Colombia. With this certification, INVIMA ensures that only qualified personnel handle your products, they are stored at the right temperature, and that the storage facility has standard operating procedures to ensure quality and safety in their internal processes.
Can bioaccess™ be my company's temporary importer of record (IOR) while I find a permanent IOR/distributor in Colombia?
Yes! bioaccess™ can become your temporary IOR while you find a permanent IOR/distributor in Colombia. Read more.
What are the post-market technovigilance controls for medical devices in Colombia?
Decree 4725 of 2005 and Resolution 4816 of 2008 set forth requirements for tracking medical devices. Medical device reporting is required by Decree 4725 which mandates all stakeholders who become aware of adverse events to notify INVIMA. Additionally, INVIMA requires complete resolution and notification of any correction, removal, or recall. Typically, the foreign manufacturer delegates this reporting requirement to either its regulatory registered agent/legal representative, its importer of record, or one of its distributors in Colombia.
This summarizes Colombia’s technovigilance program:
Manufacturers or importers must report serious and non-serious adverse events to INVIMA.
Manufacturers or importers must report international alerts to INVIMA.
Manufacturers or importers must report non-serious adverse event reports every three months.
Manufacturers, importers, or healthcare providers must report serious adverse event reports within 72 hours after its occurrence.
If your company appoints us as its regulatory registered agent/legal representative in Colombia, we can take charge of this requirement by billing (at our regulatory consulting rate) your company for the time involved per the adverse event report submitted to INVIMA. If you have more than one distributor in Colombia, your company certainly would want to centralize compliance of the reporting requirement with an independent third party such as its regulatory legal representative.
Medical device registration title holders of INVIMA-classified Class IIb and III devices must also submit to INVIMA (with the help of their local regulatory registered agent/legal representative) an annual report (Article 24, Decree 4725 of 2005) stating:
Number of imported devices
Number of sold devices
Location of all sold devices
Serial number of all sold devices
Serious adverse events and the actions that were taken
Source: ABC de Dispositivos Médicos (INVIMA), INVIMA presentations (in Spanish) here, and Resolution 4816 of 2008, INVIMA presentation (in English) here.
How do I renew a product registration certificate?
You can submit an application for renewal 90 days before the expiration date of your registration certificate. Once a product registration certificate expires, then you will have to re-submit a new application.
How do I confirm whether a company has been issued an INVIMA registration certificate as they are claiming they have been so approved?
Please visit INVIMA’s website (in Spanish) for that information here. Alternatively, you can ask the business to send you a copy of the “registro sanitario” (market approval certificate).
What if my company wants to amend (e.g. new importer of record, new manufacturer, new registration holder, product name, new label, use indication, risk classification, etc.) its existing registration certificate?
It will be easily done after submitting a change request to INVIMA. INVIMA will take about 30 days to make the change after the submission of the application. We will invoice you for our regulatory consulting time plus any applicable INVIMA fee. Click here to see our price list.
Can my company sell directly to end-users in Colombia?
Yes, as long as your end-user (buyer) contacts your IOR in Colombia to process the physical importation of your medical device. Bear in mind that your IOR may be a logistics operator or a commercial distributor.
We have a distributor that registered our products at INVIMA in Colombia. Can we re-register the same products at INVIMA? If not, can we transfer the registration to our company (no office in Colombia)?
Yes, you can re-register the same products at INVIMA. If you want to transfer the registration that your past distributor had to your foreign company, then you must get a written authorization from your distributor. You can hold an INVIMA registration under your foreign company's name; you don't have to have an office in Colombia.
bioaccess™ importer of record (IOR) service allows foreign medical device and IVD companies to expand into Colombia with ease. Our IOR service simplifies the market clearance of your medical product at INVIMA and saves you from the costs of establishing a legal entity or from waiting to find a suitable distributor.
Importing/Nationalizing Products into Colombia
What's the process to obtain an import permit to ship products and import them into Colombia?
Once INVIMA issues your registration certificate, Colombia’s Ministry of Industry and Commerce (MinCIT) is the entity that issues import permits. You will need to provide MinCIT with a proforma invoice of the shipment, the INVIMA registration certificate, and complete an electronic application. You will need a separate import permit for each shipment.
How do I express-ship urgent medical devices to Colombia?
Sometimes manufacturers urgently need to ship medical devices to their distributors or clients. INVIMA doesn't allow the importation of unregistered medical devices sent by courier (i.e., DHL, FedEx, UPS, etc) into Colombia. INVIMA and the Colombia customs agency (DIAN) work closely and together inspect and process courier shipments of regulated products at airports.
You won’t be able to express-ship demo units of INVIMA-unregistered medical devices to Colombia. The medical devices that you intend to ship to Colombia must first have market clearance in Colombia in the form of an INVIMA-issued registration certificate that covers the references, models, brand, and commercial presentation of the device.
There are two ways to do quickly import regulated products (previously registered at INVIMA) into Colombia:
Trafico postal y envíos urgentes or postal traffic and urgent shipments (chapter 12, article 254 of Decreto 1165 de 2019). You will be able to easily ship demo units or other accessories to your distributor or client in Colombia by express courier (i.e. DHL, FedEx, UPS). These are the restrictions for express shipments to Colombia:
The declared value cannot exceed USD 2,000.
The weight cannot exceed 50 kilograms (110.23 pounds).
The goods must not be subject to legal or administrative restrictions.
The goods must not include weapons, drugs, or any other product that is subject to international shipping restrictions.
No more than six (6) units of the same product.
The shipping box size cannot exceed 1.5 meters (59.05 inches).
Muestras sin valor comercial or samples with no commercial value (chapter 4, article 192 of Decreto 1165 de 2019): DIAN, Colombia’s custom agency, regulates how samples with no commercial value are imported into Colombia. You should consult with your receiver in Colombia about this import modality. Your receiver in Colombia may need to hire the services of a customs broker to process the importation of product samples with no commercial value.
Note: Duties and taxes on the trafico postal y envíos urgentes import modality are usually billed automatically to the recipient unless the shipper specifically requests that the courier bills the shipper or a third party. When completing your courier Waybill, please select the shipper (you) as the party responsible for payment of taxes. There are the taxes that you should expect to pay under this import modality:
Import duty: 10% of the CIF (cost, insurance, and freight) value
Sales tax: 19% of CIF value + import duty
No taxes if the declared value is less than USD 200
I shipped demo regulated products to someone in Colombia and Colombian customs (DIAN) is holding the shipment. What should I do?
The first thing you must find out is the exact reason why DIAN is holding the shipment. The recipient of the shipment in Colombia should have received a letter from DIAN similar to this one here. There could be several reasons for the hold:
You shipped a regulated product that lacks an INVIMA-issued registration certificate.
The shipment lacks a commercial invoice or the invoice is incorrect.
The import modality was poorly chosen by the shipper.
DIAN determined that the shipment doesn't qualify to be imported under the import modality that wants the recipient to switch import modalities, etc.
The most likely reason for the hold is that DIAN determined that the shipment doesn't qualify to be imported under the trafico postal and envíos urgentes import modality (it didn't meet the restrictions outlined in our answer above (chapter 12, article 254 of Decreto 1165 de 2019).
Every time you ship goods into a country, you have to speak with the recipient and agree on what import modality your shipment will be nationalized under. Once you choose your import modality, then you should ship the goods following the restrictions and requirements for that specific modality.
You have a limited number of days (30-60) to remedy this situation. Otherwise, the shipment will be considered abandoned.
Switching the import modality to “ordinary” will likely not work since the recipient won’t be able to nationalize the products until the product has the proper INVIMA registration certificate. “Ordinary” importation requires that the goods have and INVIMA certificate. Besides, the importer of record will likely not be the recipient you sent this samples to. The importer of record listed in the INVIMA certificate is the only company allowed to import the medical product listed in the certificate. This right cannot by temporarily transferred to a third-party just for one single importation.
Usually, this type of holds are handled locally between the recipient and the courier/transportation company and/or the recipient's customs broker. After the recipient's speaks with either of these two parties, then the recipient will tell you what you can do from your end to resolve it.
The recipient should speak with the courier and/or its custom broker to see whether it can nationalize the shipment under another modality of importation: Muestras sin valor comercial. This may be the most suitable import modality at this juncture. The recipient won't be able to nationalize the shipment under the trafico postal y envíos urgentes import modality. The recipient has to become creative with its custom broker to try to release the shipment under another import modality. Muestras sin valor comercial is just another import modality that allows the importation of samples with no commercial value. After the tráfico postal y envíos urgentes import modality, it is the most simplified way to import demo/sample products into the country.
There is nothing you can do do from your end at this point except telling the recipient to speak with the courier and/or its custom broker about the situation and see if the custom broker sees a way to try to release the shipment under the muestras sin valor comercial import modality. If the recipient is a company and is experienced in importing goods into Colombia, then it should be already working with a customs broker. If the recipient is not an experienced importer, then there isn't really much you can do to nationalize the shipment into the country, and the best option is just to abandon the shipment or to speak with your courier to see if it can be returned back to its place of origin.
Regardless of what you do, after the 30-60 days lapse, the recipient must pay penalties, hefty storage fees, hire the services of a customs broker, etc. It’s very likely that the fees associated with releasing this shipment surpass the value of the goods. Unless the recipient with the help of its customs broker can switch the import modality to muestras sin valor comercial, then you should just abandon this shipment and take a loss.
Regardless of the reason for the hold, this is what we suggest you do next:
Ask your recipient to share the DIAN letter with you so that you know exactly what was the reason for the hold.
Ask your recipient to contact the shipping company if he/she doesn’t have the DIAN letter to ask for a copy of it.
If the reason for the hold is because the goods were incorrectly shipped under the tráfico postal y envíos urgentes modality, then agree with the recipient to switch import modality (e.g., from the tráfico postal y envíos urgentes modality to the muestras sin valor comercial modality or the ordinary importation modality). For this, the recipient must seek the services of an import broker to process this importation.






